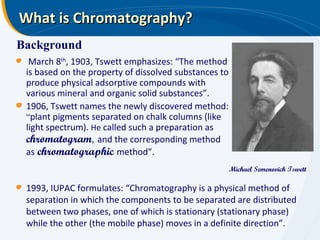
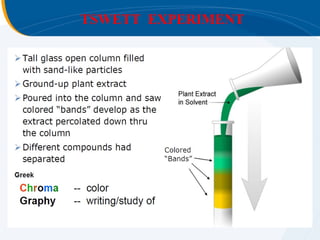
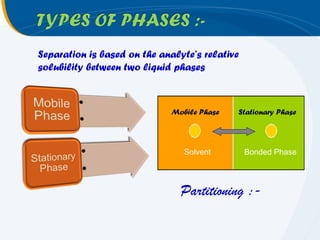
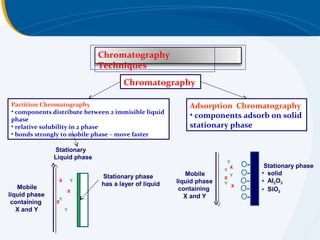
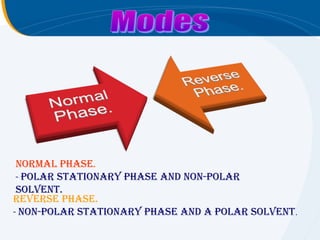
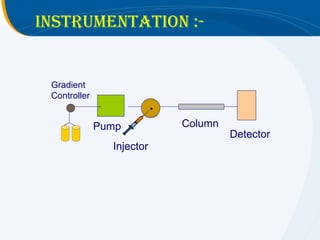
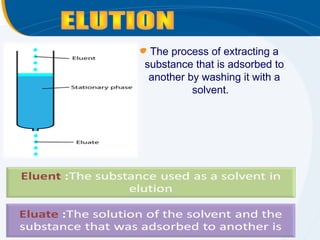
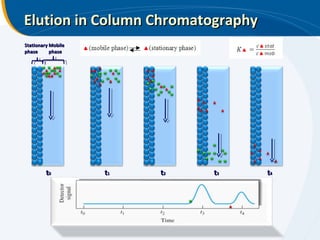
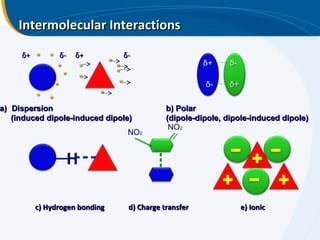
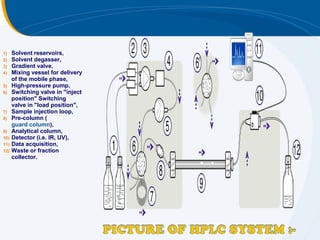
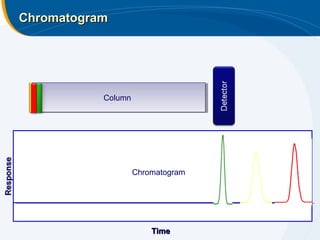
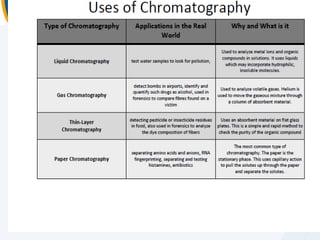
The presentation outlines the principles and applications of liquid chromatography, tracing its history from Tswett's initial discoveries to modern techniques like HPLC and GC. It emphasizes the separation of chemical components based on their interactions with stationary and mobile phases, detailing various chromatography techniques and the role of instrumentation. The conclusions highlight the utility of liquid chromatography for analyzing non-volatile and thermally unstable substances.