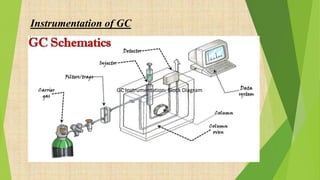
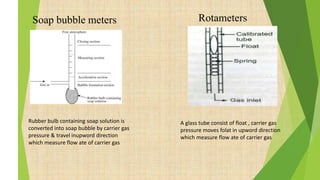
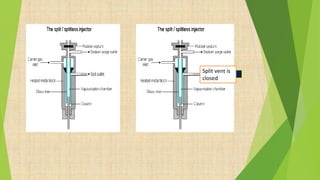
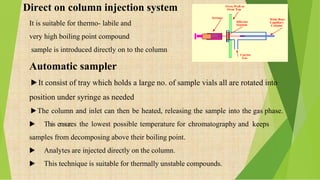
The document provides information about gas chromatography (GC). It begins with definitions, stating that GC separates compounds based on their volatility between a stationary and mobile gas phase. The basic principle is that a sample is vaporized and injected into a column, where components are separated as they are eluted by an inert gas and detected. Key components of a GC system are described, including the carrier gas, injector, column placed in an oven, and various detectors. Common detectors mentioned are the flame ionization detector and electron capture detector. The document also discusses the process of derivatization used to modify compounds to make them more suitable for GC analysis by increasing volatility or detectability.






























![4. Chiral derivatization : -
These reagents target one specific functional group and produce individual diasteriomers
of each of the enantiomers. There are two ways to separating enantiomers by
chromatography:
I. Separation on an optically active stationary phase.
II. Preparation of diastereomeric derivatives that can be separated on a non stationary
phase.
REAGENTS : -
A. TPC (N-trifluroacetyl-L-prolyl chloride)
Used for optically active amines, most notable amphetamines.
B. MCF [(-)methylchloroformate]
Used for optically active alcohols.
- If an optically pure reagent is used to prepare diasteriomeric derivatives, then only two
derivatives are formed. The enantiomeric ratio is reflected in the relative peak size.](https://image.slidesharecdn.com/gcppt-230405171134-57c04582/85/GC-PPT-pptx-35-320.jpg)




