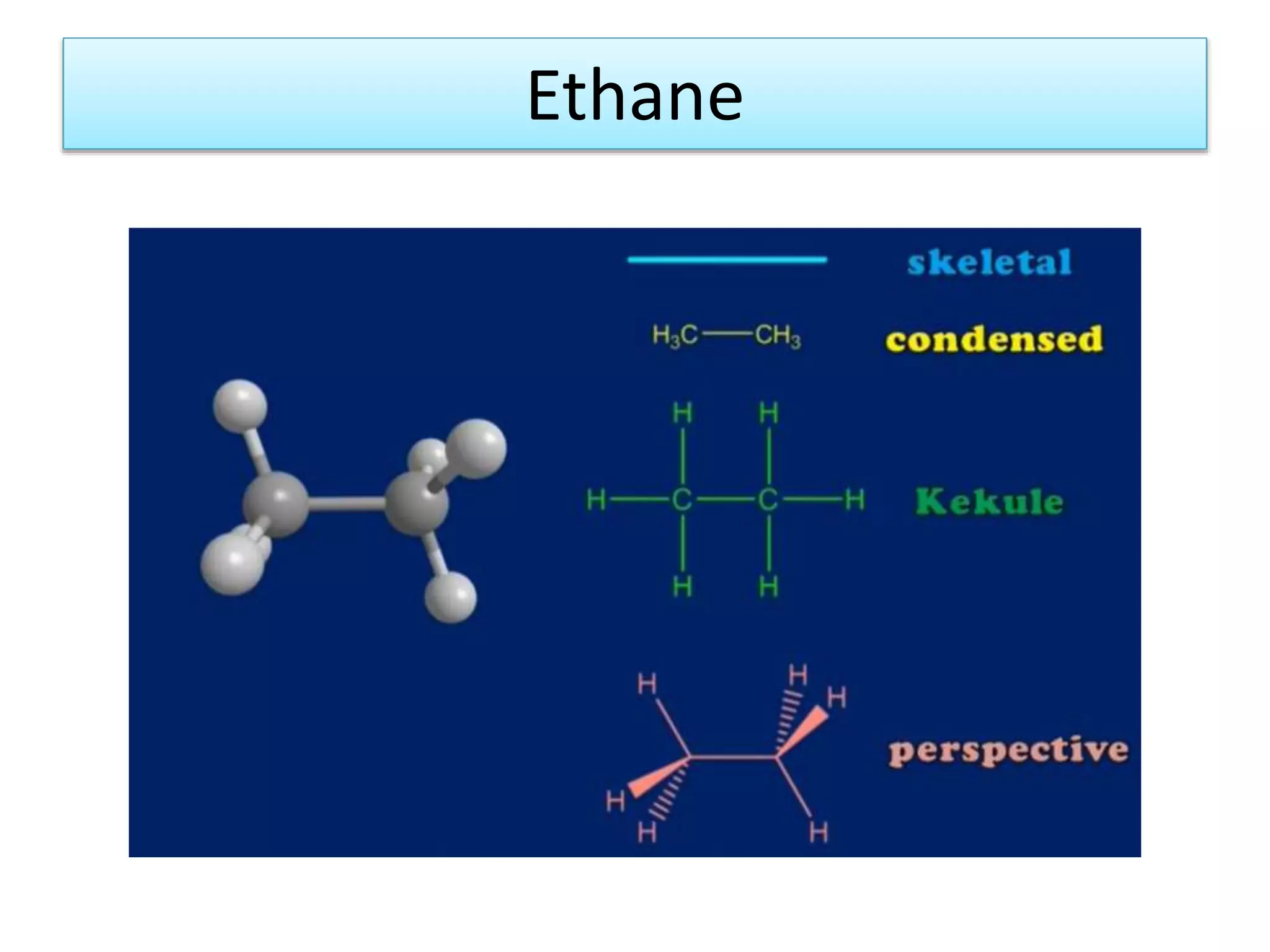
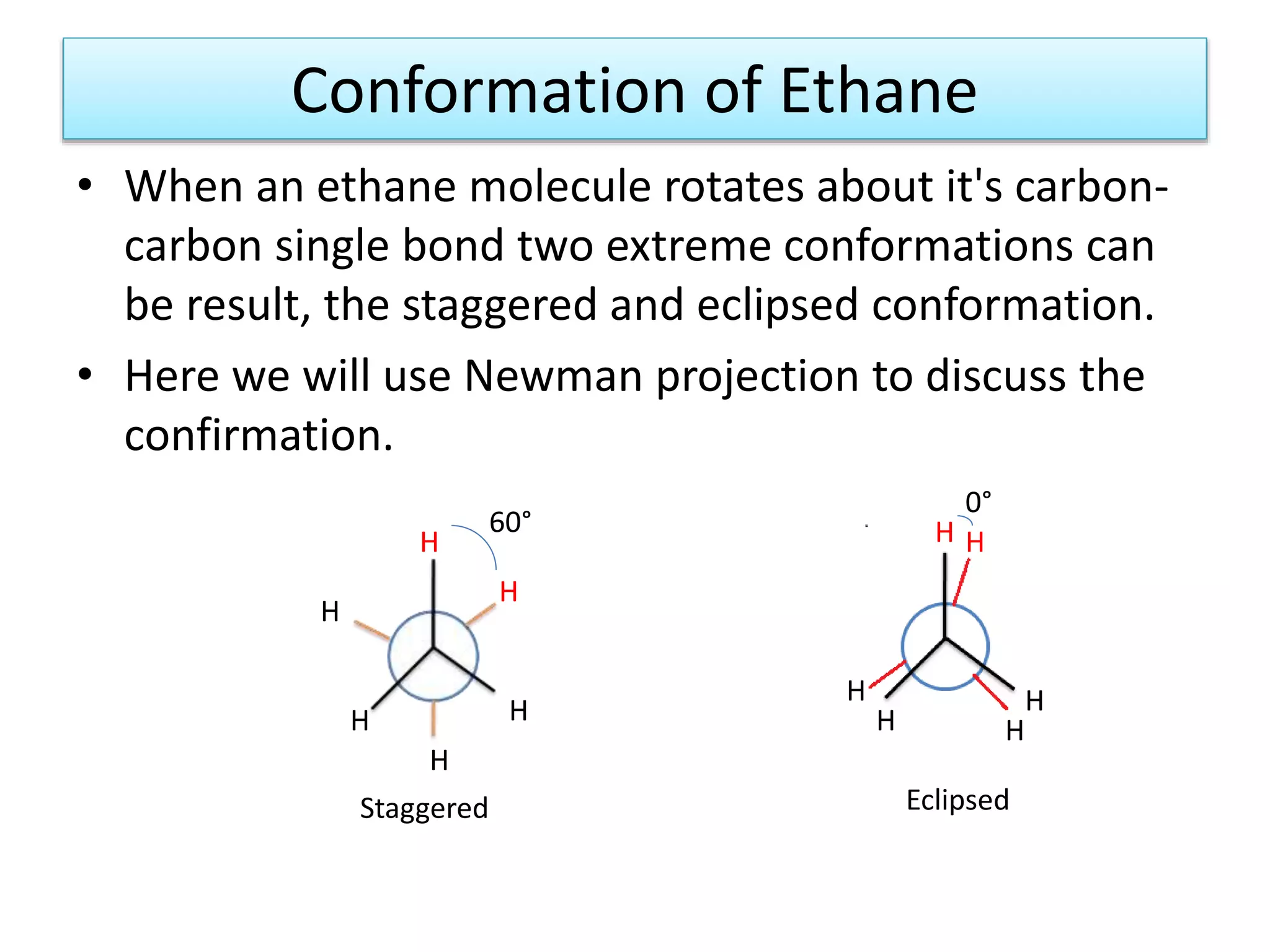
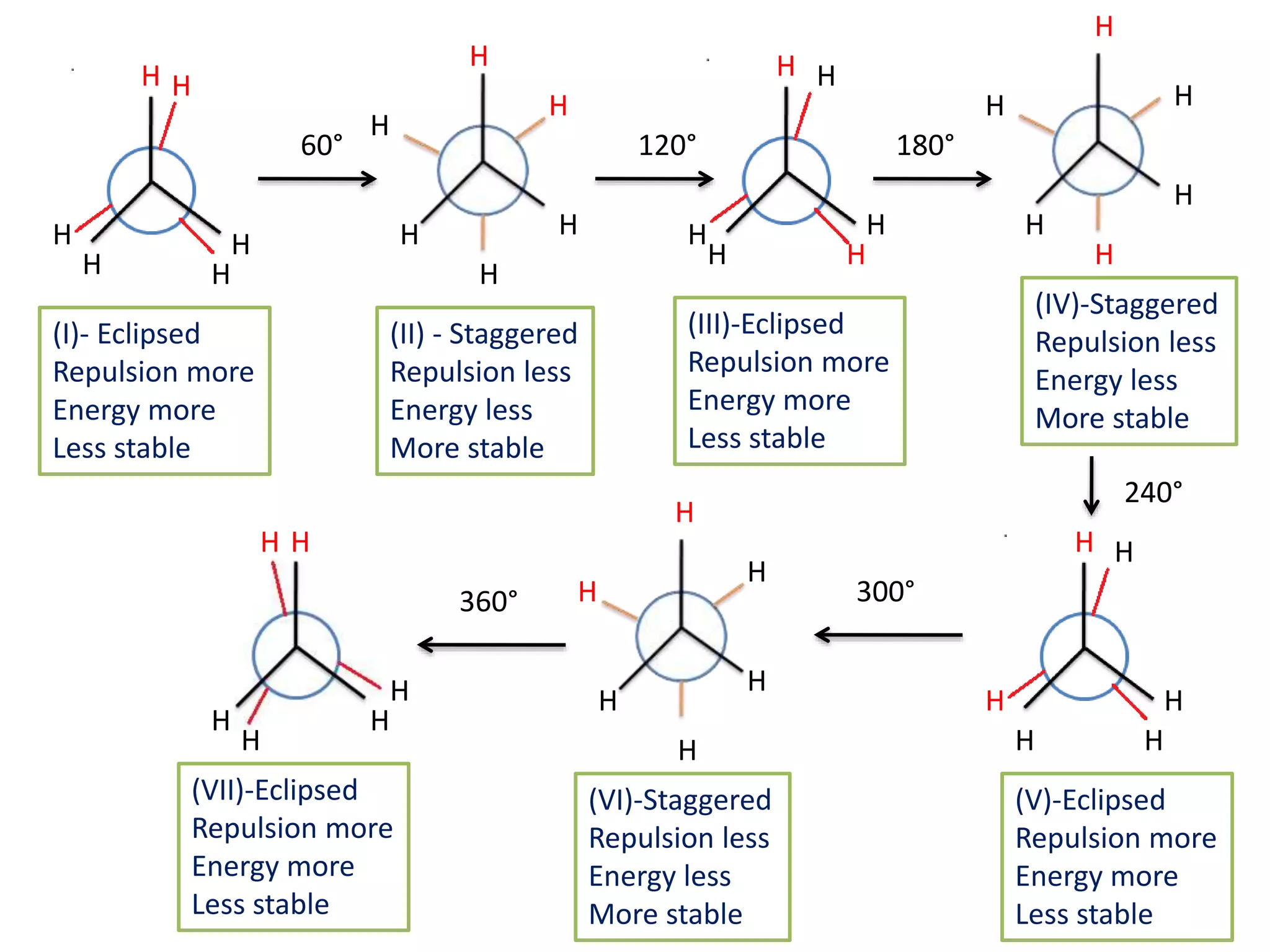
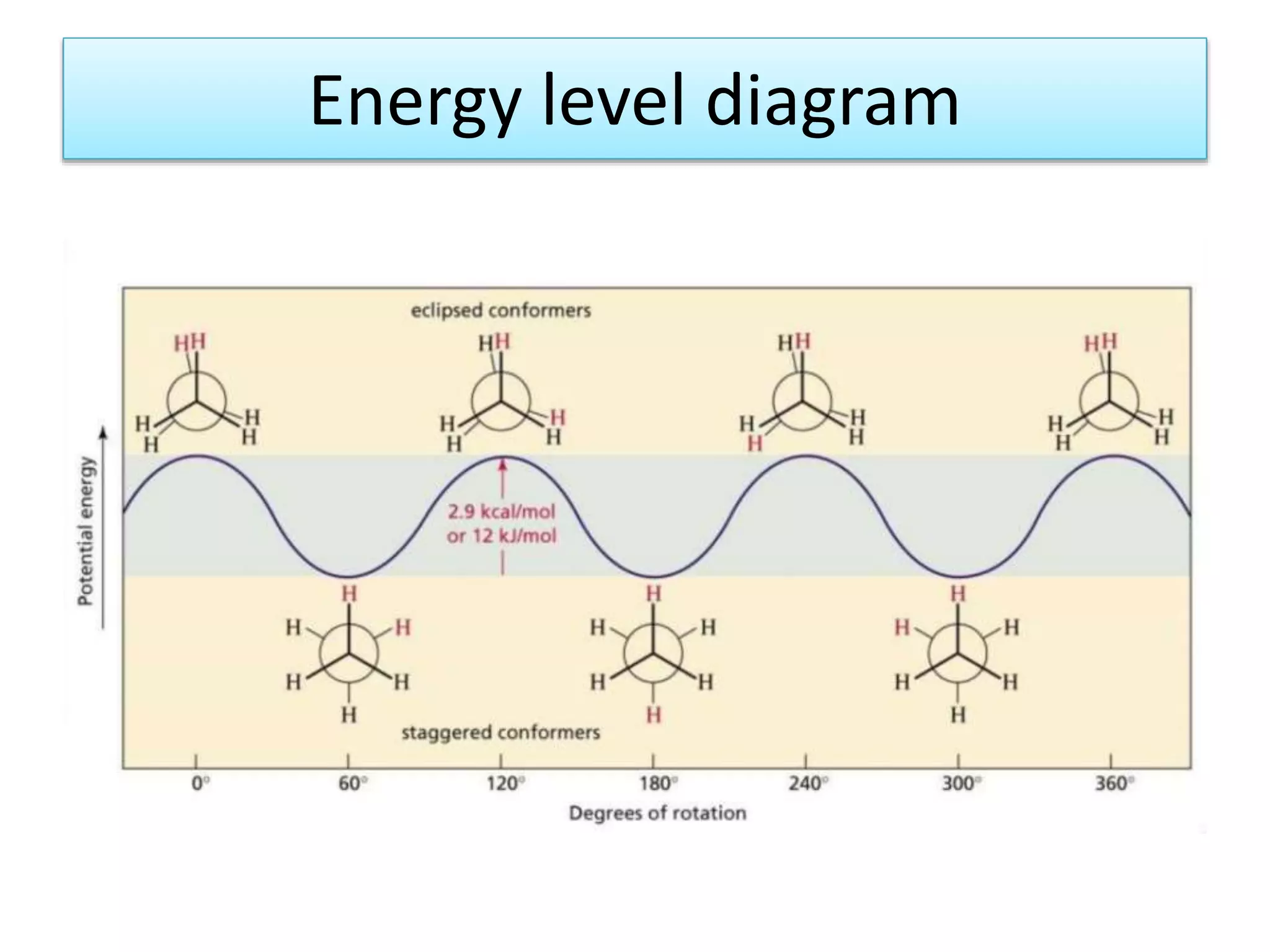
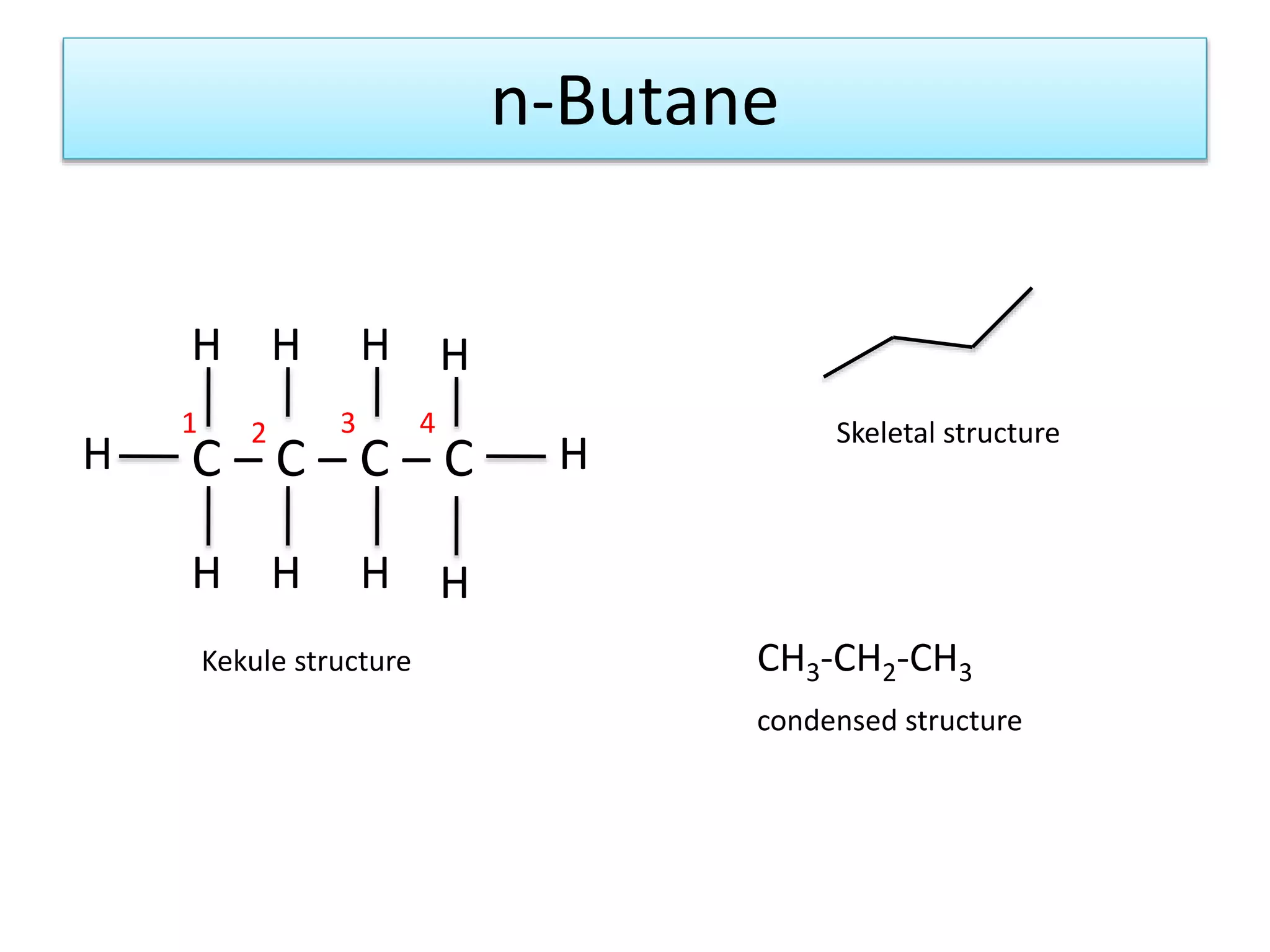
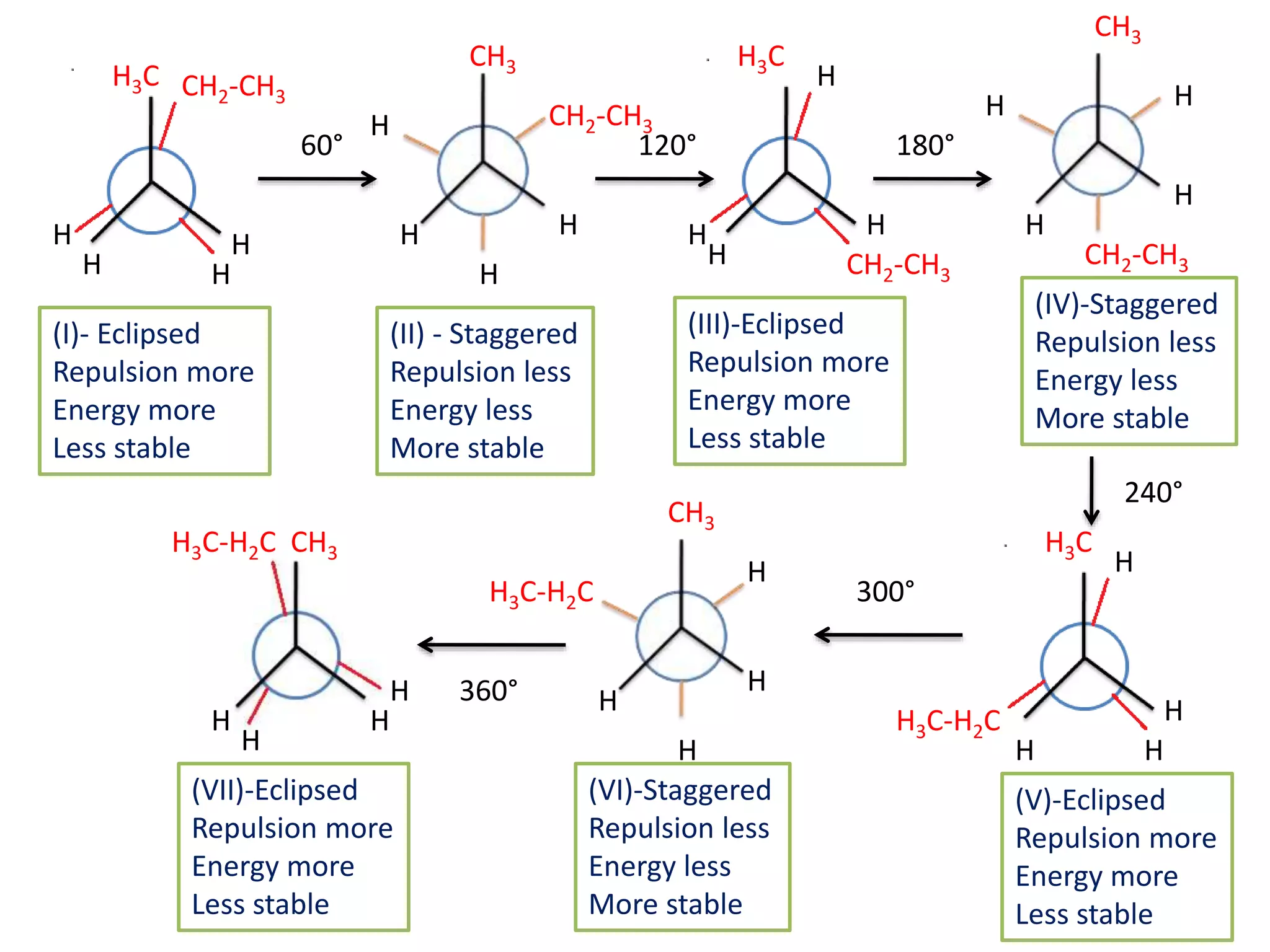
The document discusses the conformations of ethane and butane molecules. It explains that ethane can exist in staggered or eclipsed conformations, with the staggered being more stable due to less repulsive interactions. Six conformations are possible when one methyl group rotates, with three staggered and three eclipsed. Staggered conformations have lower potential energy. Butane also has staggered and eclipsed conformations, with the anti conformation being the most stable due to the methyl groups being furthest apart.