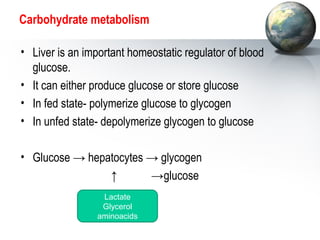
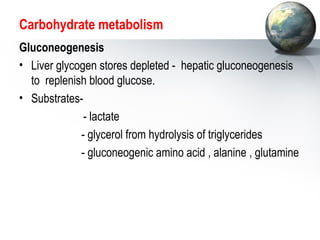
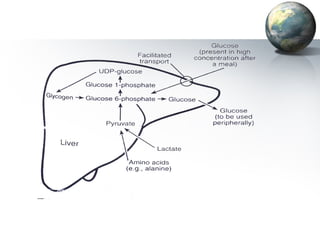
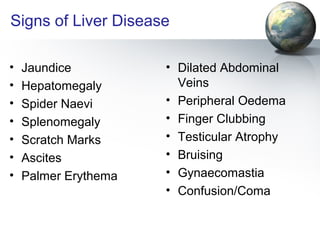
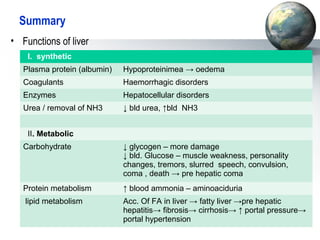
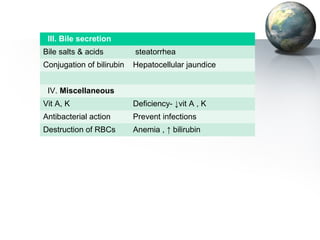
The liver performs many important physiological functions:
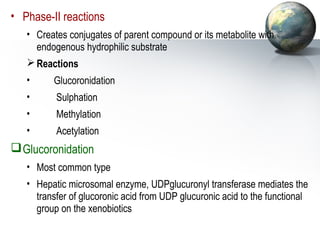
1. It acts as the epicenter of intermediary metabolism, performing diverse biochemical pathways related to carbohydrate, lipid, protein, and xenobiotic metabolism.
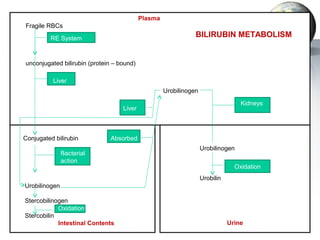
2. The liver synthesizes proteins involved in blood coagulation, stores vitamins and iron, and breaks down bilirubin.
3. The liver's endocrine functions include modifying hormone action and producing insulin-like growth factors. Its versatile roles impact every tissue in the body.