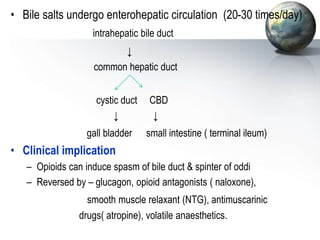
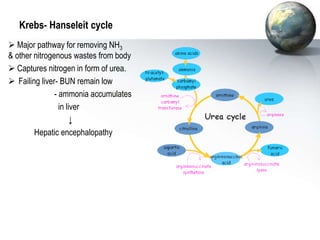
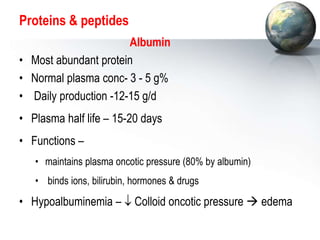
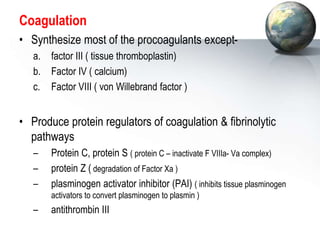
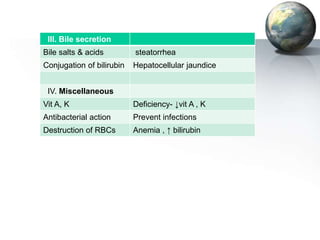
The liver performs many critical physiological functions:
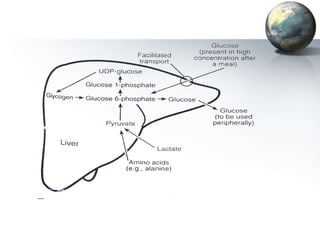
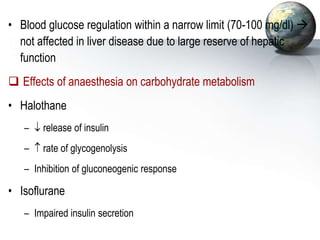
1. It regulates carbohydrate, lipid, and protein metabolism, producing glucose and ketone bodies and breaking down toxins.
2. The liver synthesizes proteins involved in blood clotting and transports iron, vitamins, and hormones.
3. The liver metabolizes and detoxifies drugs and other xenobiotics through phase I and phase II reactions and transports them out of the body. Impairment of these functions can lead to drug accumulation and toxicity.