The fuel-air cycle provides a more accurate model of the actual thermodynamic cycle in an internal combustion engine compared to the air standard cycle by accounting for:
1) The actual composition of gases in the cylinder, which varies throughout the cycle.
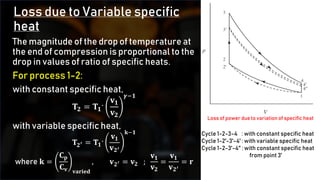
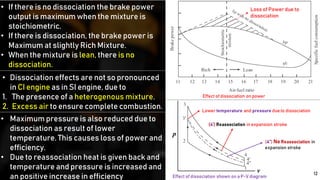
2) Variations in specific heat and dissociation effects at high temperatures.
3) Changes in the number of moles as pressure and temperature fluctuate.
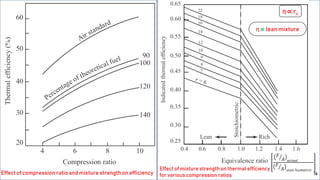
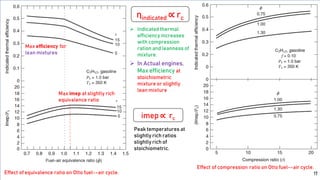
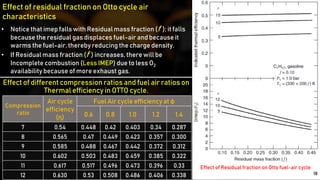
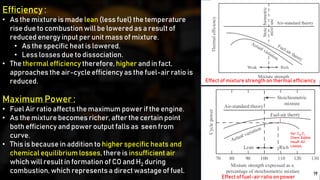
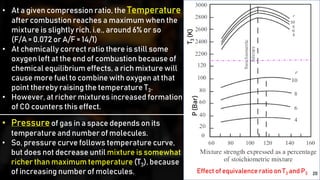
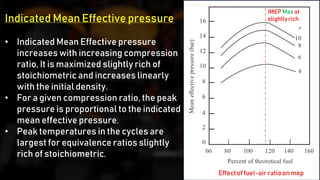
The fuel-air cycle shows that efficiency is maximized with a slightly rich mixture near stoichiometric due to higher temperatures from dissociation. It also demonstrates efficiency gains from higher compression but losses from richer mixtures beyond stoichiometric due to incomplete combustion.