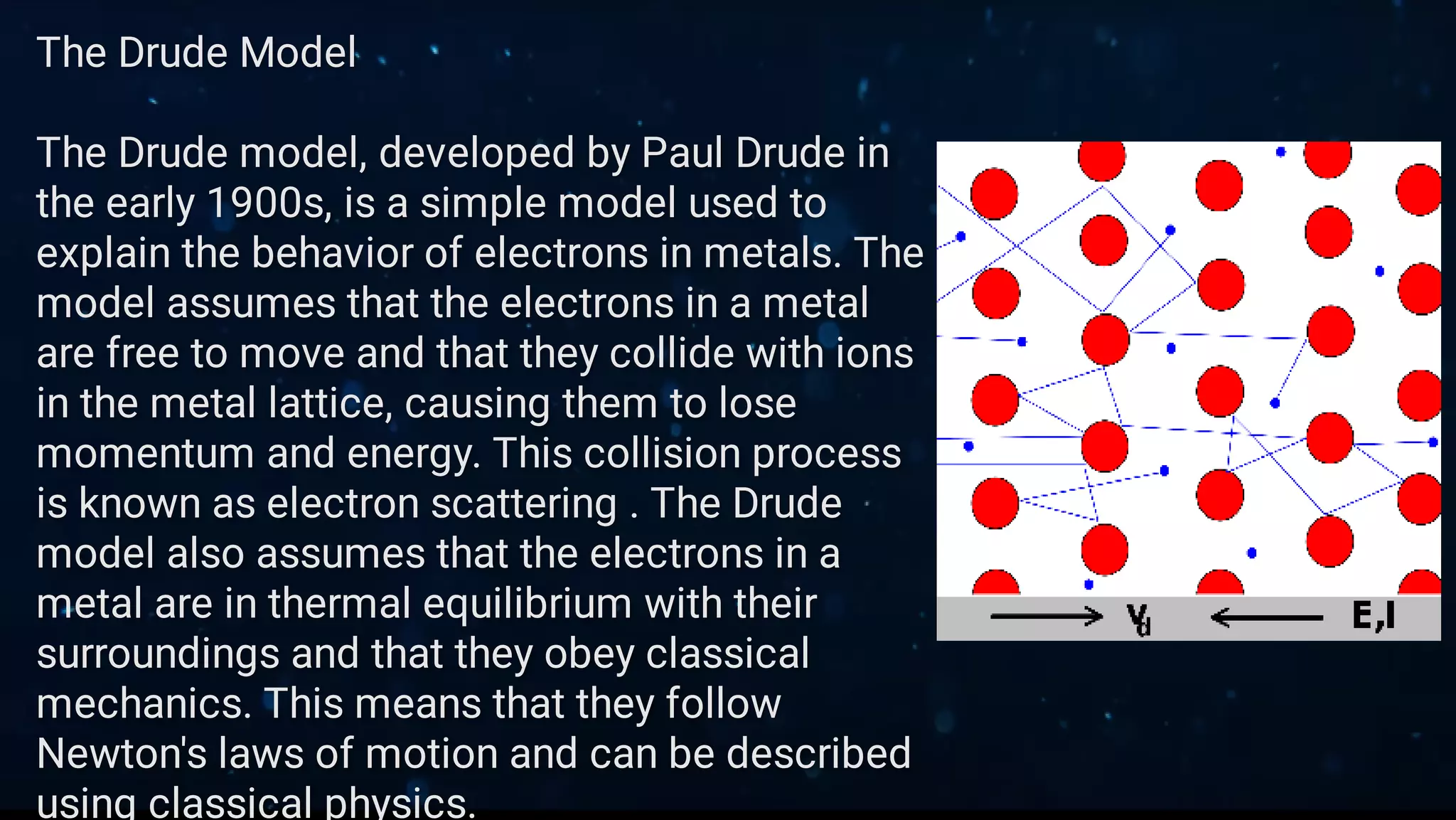
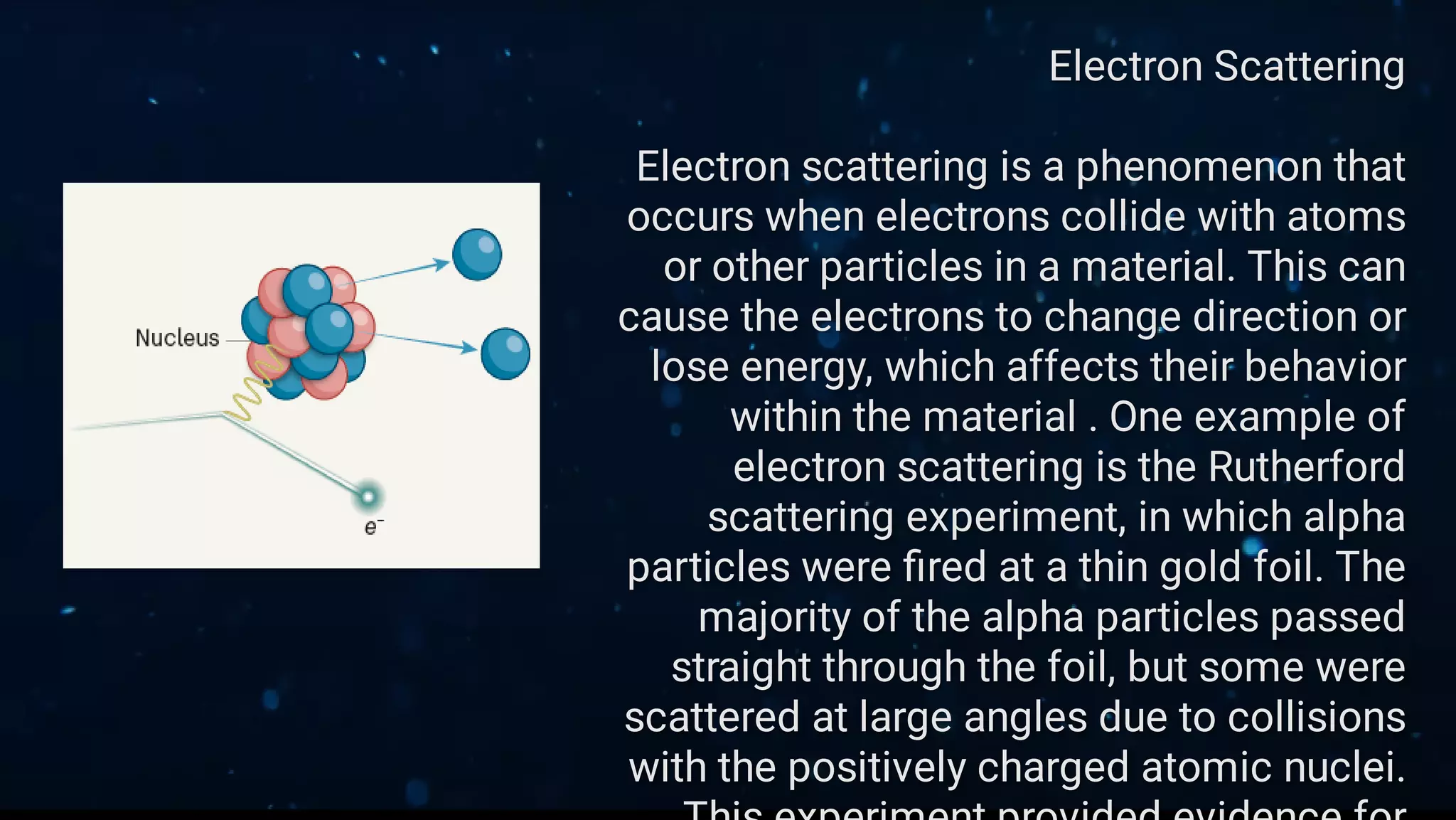
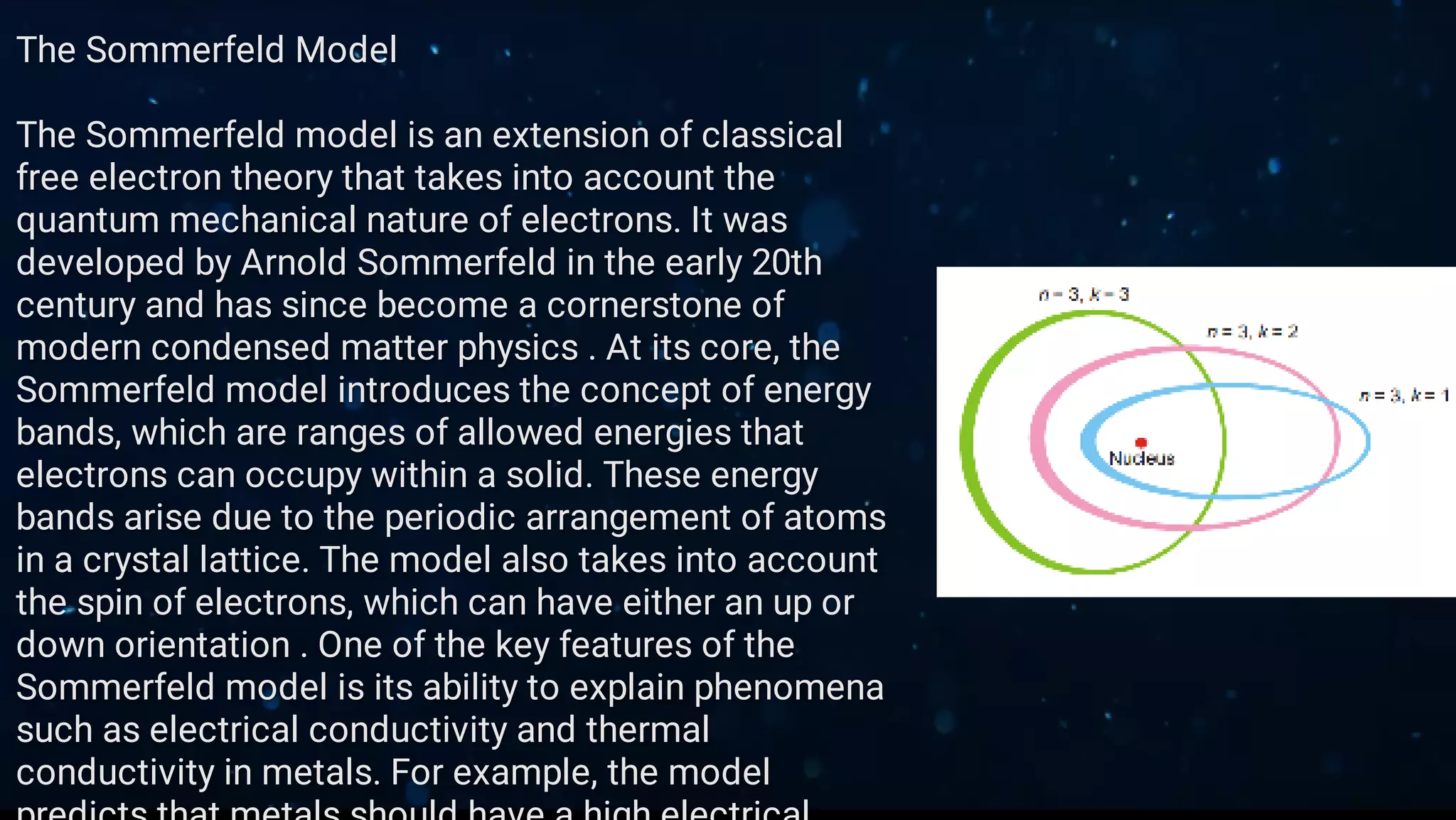
This document provides an overview of classical free electron theory, including its key concepts, models, limitations, and applications. It introduces classical free electron theory and the Drude model. It discusses electron scattering, electrical conductivity, and the limitations of the classical approach. It then covers how quantum mechanics improved the understanding of electron behavior. The Sommerfeld model incorporated quantum principles like energy bands. Finally, it discusses how classical free electron theory is applied in materials science and semiconductor development.