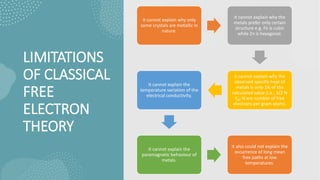
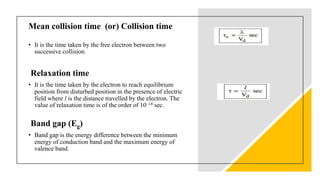
This document provides an overview of classical free electron theory, including its history, assumptions, limitations, successes, and basic terms. It was introduced in 1900 by Drude and further developed by Lorentz in 1909. The theory assumes electrons in metals move freely and collide elastically with fixed positive ions. While it could explain electrical conductivity and behavior in magnetic fields, it had limitations such as not explaining specific heat capacities and why only some materials are metallic. The advent of quantum free electron theory in 1928 by Sommerfeld addressed some of these issues and limitations.