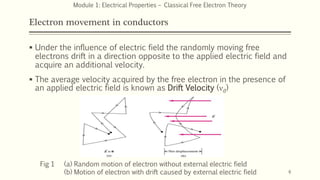
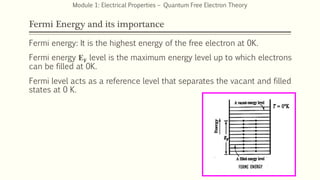
This document provides an introduction and syllabus for a module on electrical properties and free electron theory. It discusses key concepts from classical and quantum free electron theory, including postulates, failures of the classical theory, the importance of Fermi energy, and advantages of the quantum theory. Example questions are also provided to test comprehension of topics like thermal velocity, root mean square velocity, and the effects of temperature on conductivity.