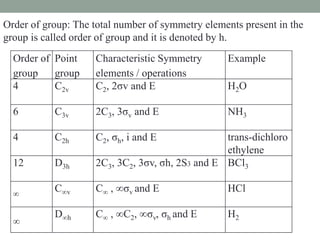
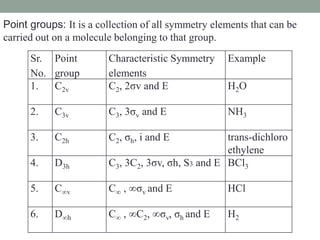
1. Point groups classify molecules based on their symmetry elements and operations.
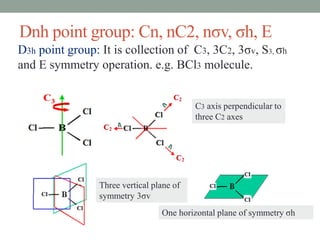
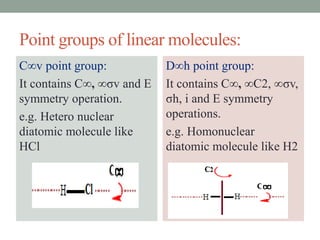
2. Common point groups include C2v, C3v, C2h, D3h, C∞v, and D∞h.
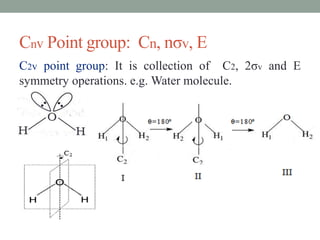
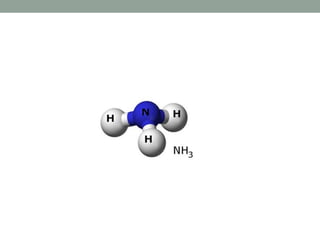
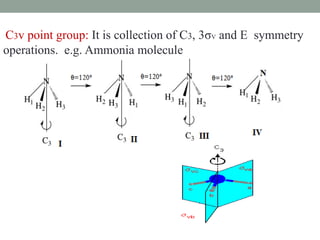
3. C2v contains C2 rotation and 2σv vertical planes of symmetry (e.g. H2O). C3v contains C3 rotation and 3σv planes (e.g. NH3).


![Classification of point groups:
Elements Point group Example
C type point group
Cn Cn H2O2
Cn + nσv Cnv H2O, NH3
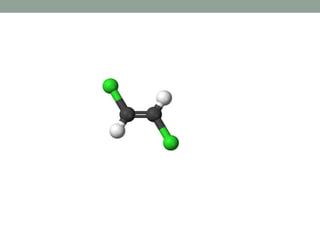
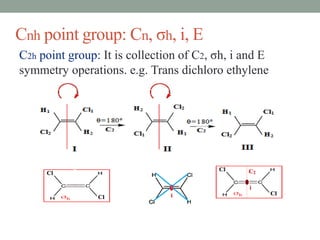
Cn+ σh Cnh Trans dichloro
ethylene
D type point group (possess Cn perpendicular to C2 axis)
Cn + nC2 Dn [Cu(en)2] +2
Cn + nC2 + nσV +
σh
Dnh BCl3
Cn + nC2 + σd Dnd Allene
Higher symmetry point groups
Tetrahedral Td CH4, CCl4
Octahedral Oh SF6](https://image.slidesharecdn.com/pointgroupspptpdf-220902152613-721e76d4/85/Point-Groups-ppt-pdf-pdf-13-320.jpg)