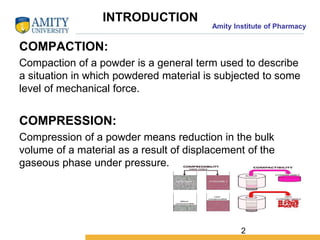
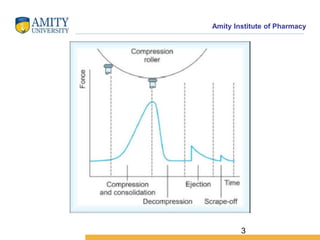
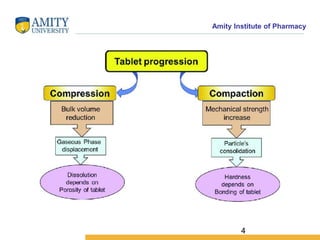
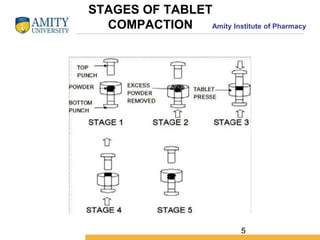
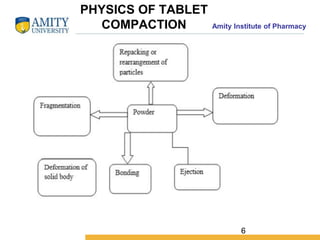
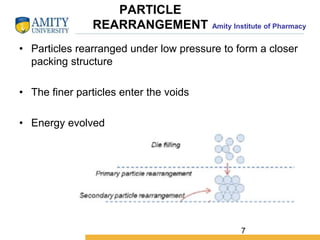
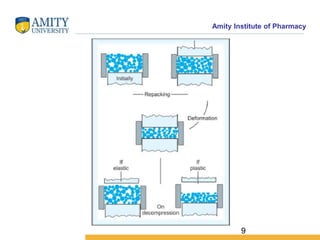
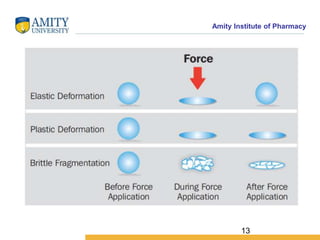
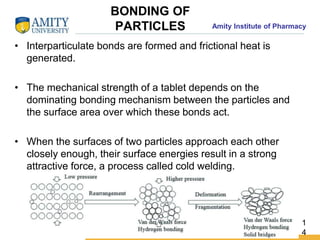
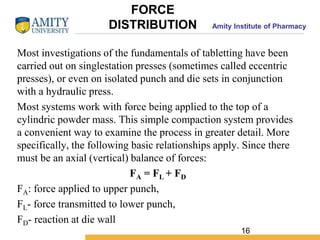
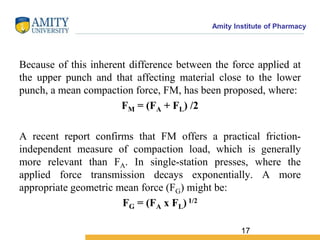
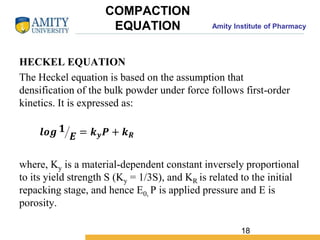
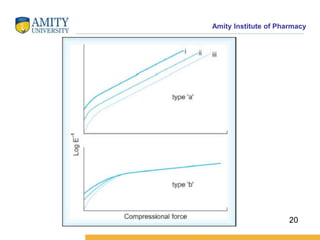
The document discusses the physics of tablet compression and compaction. It describes the stages of tablet compaction as particle rearrangement, deformation through elastic, plastic or brittle mechanisms, bonding of particles, and ejection. It also discusses force distribution during compression and the Heckel compaction equation, which models densification as a first-order kinetic process. The Heckel plot can identify the predominant deformation mechanism and correlate tablet crushing strength to material properties.