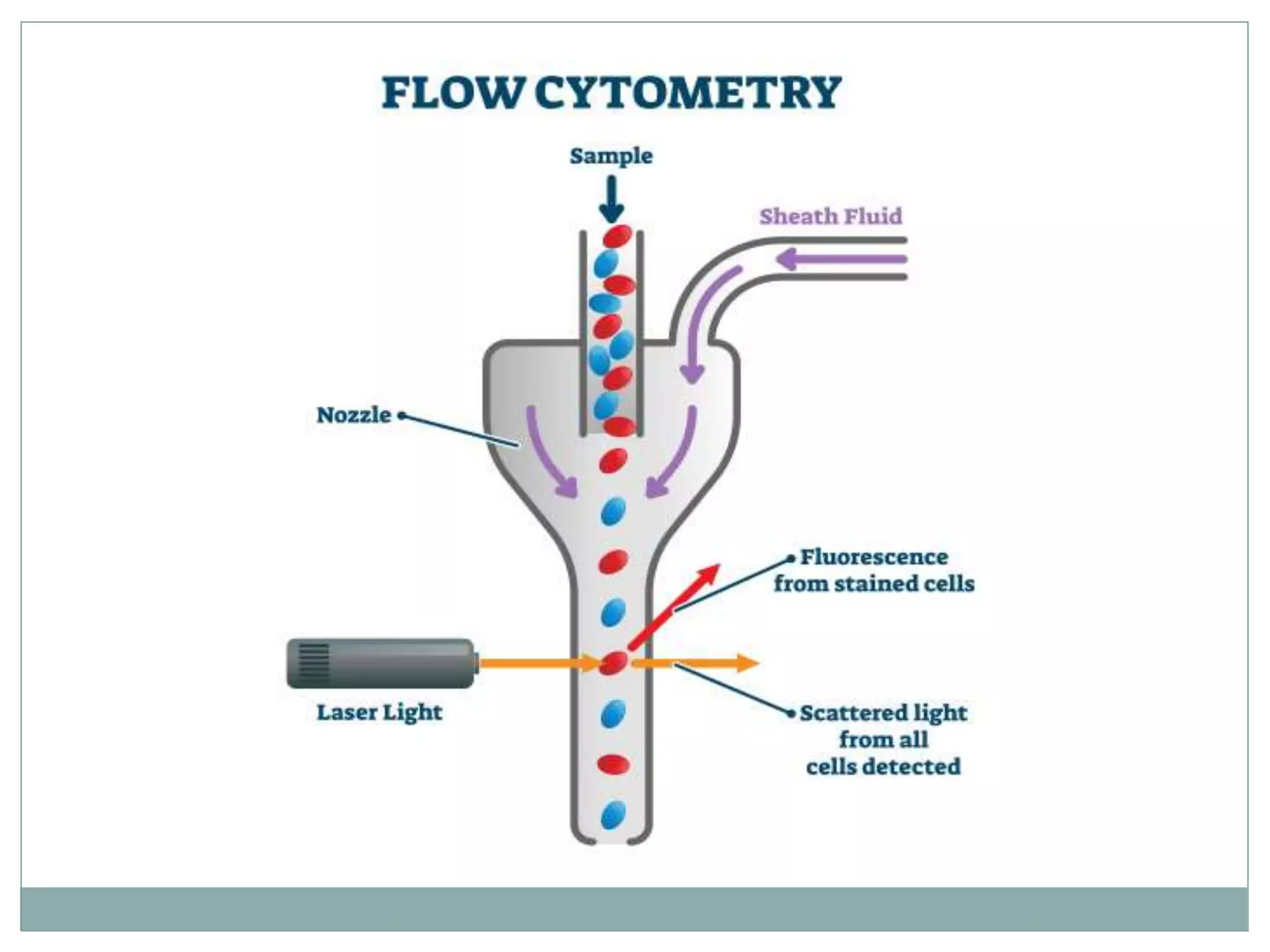
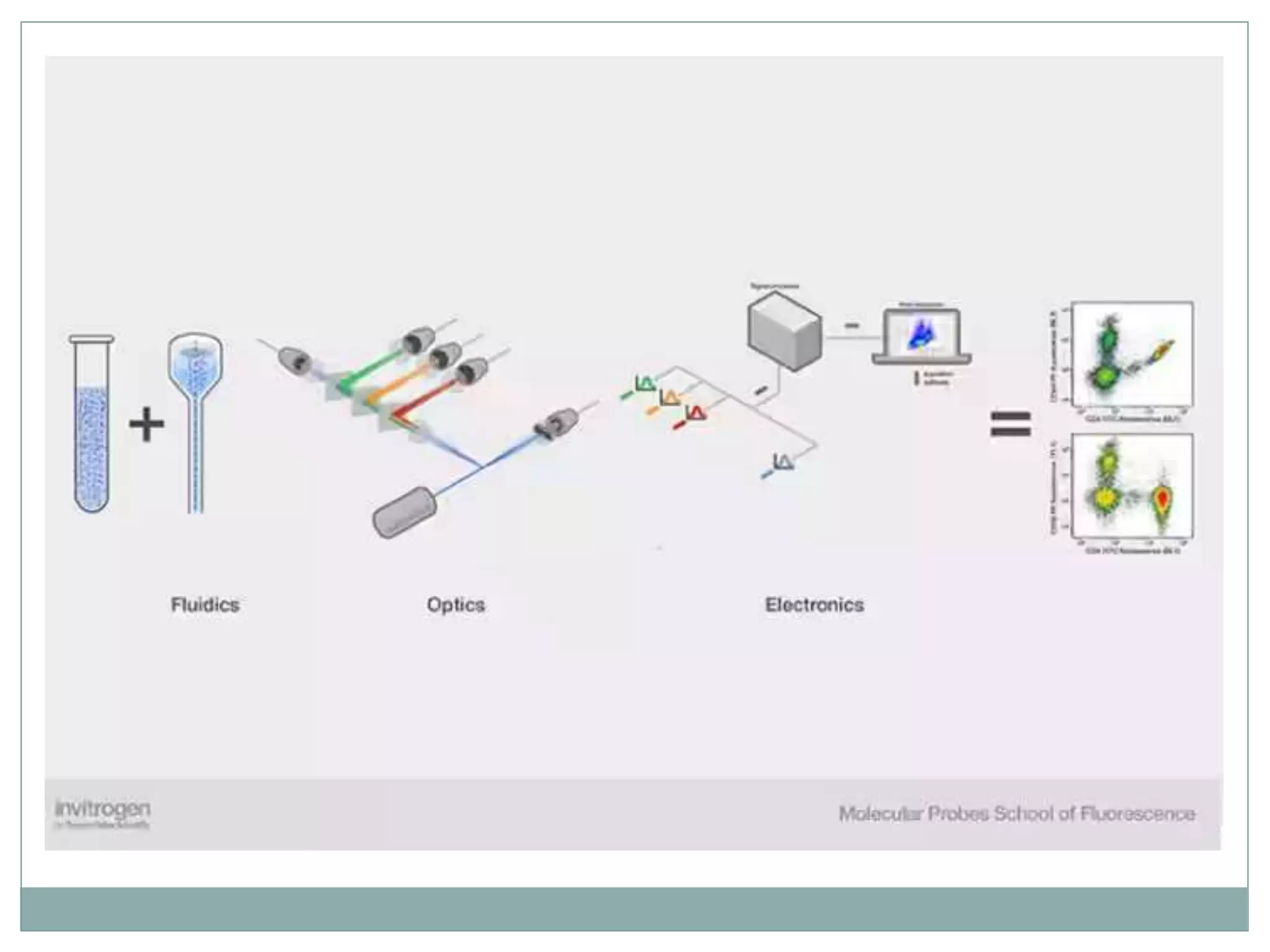
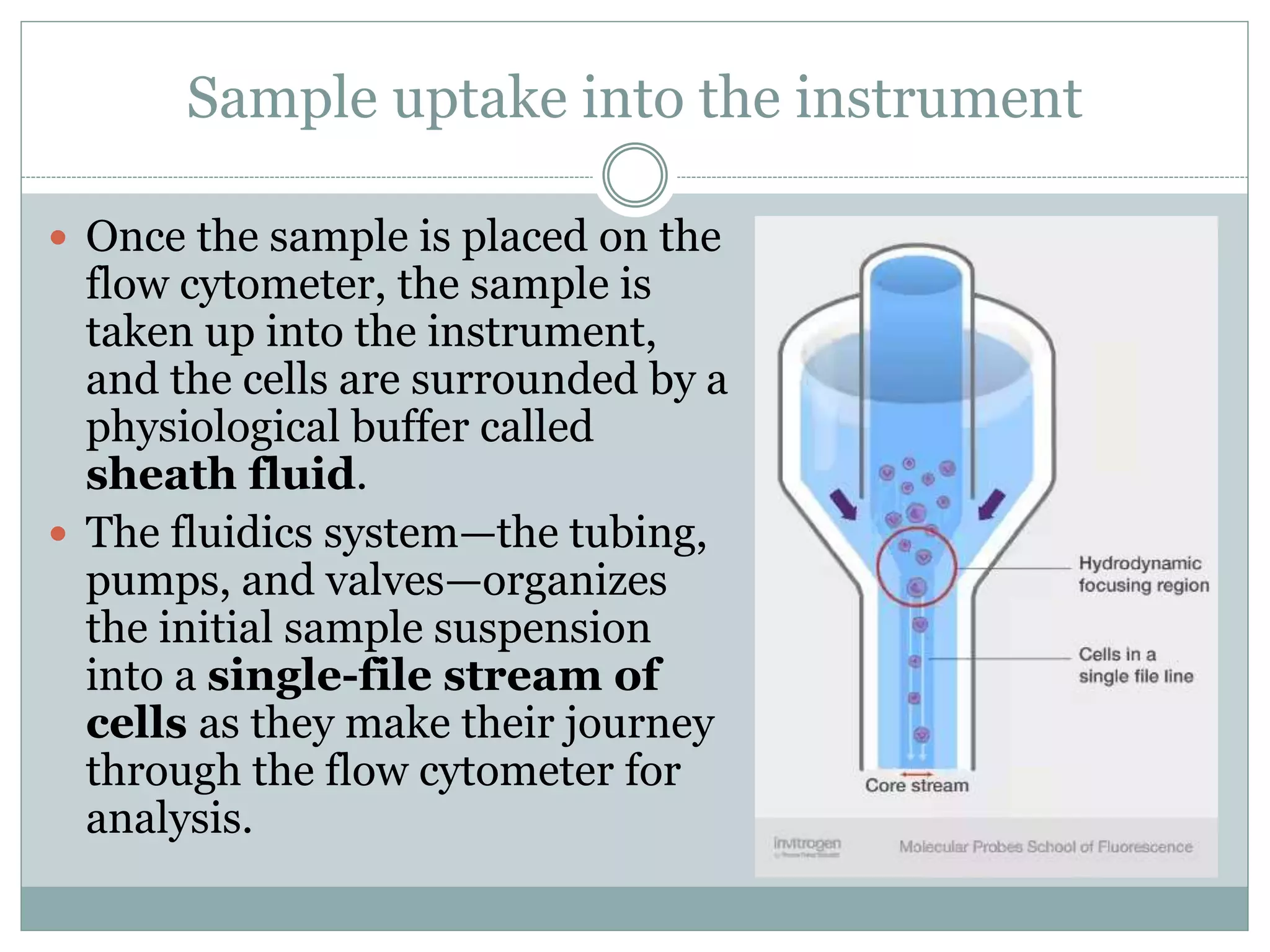
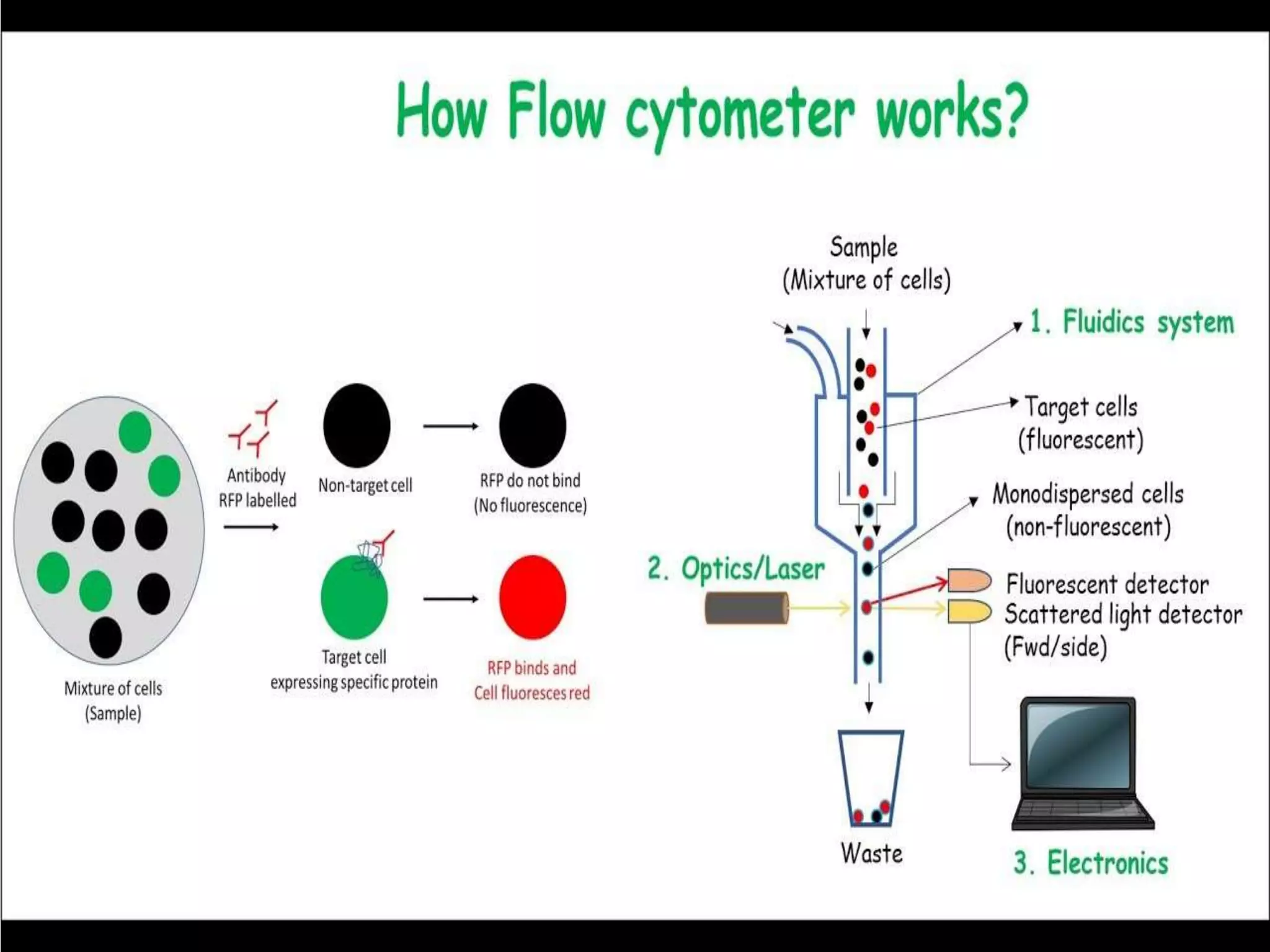
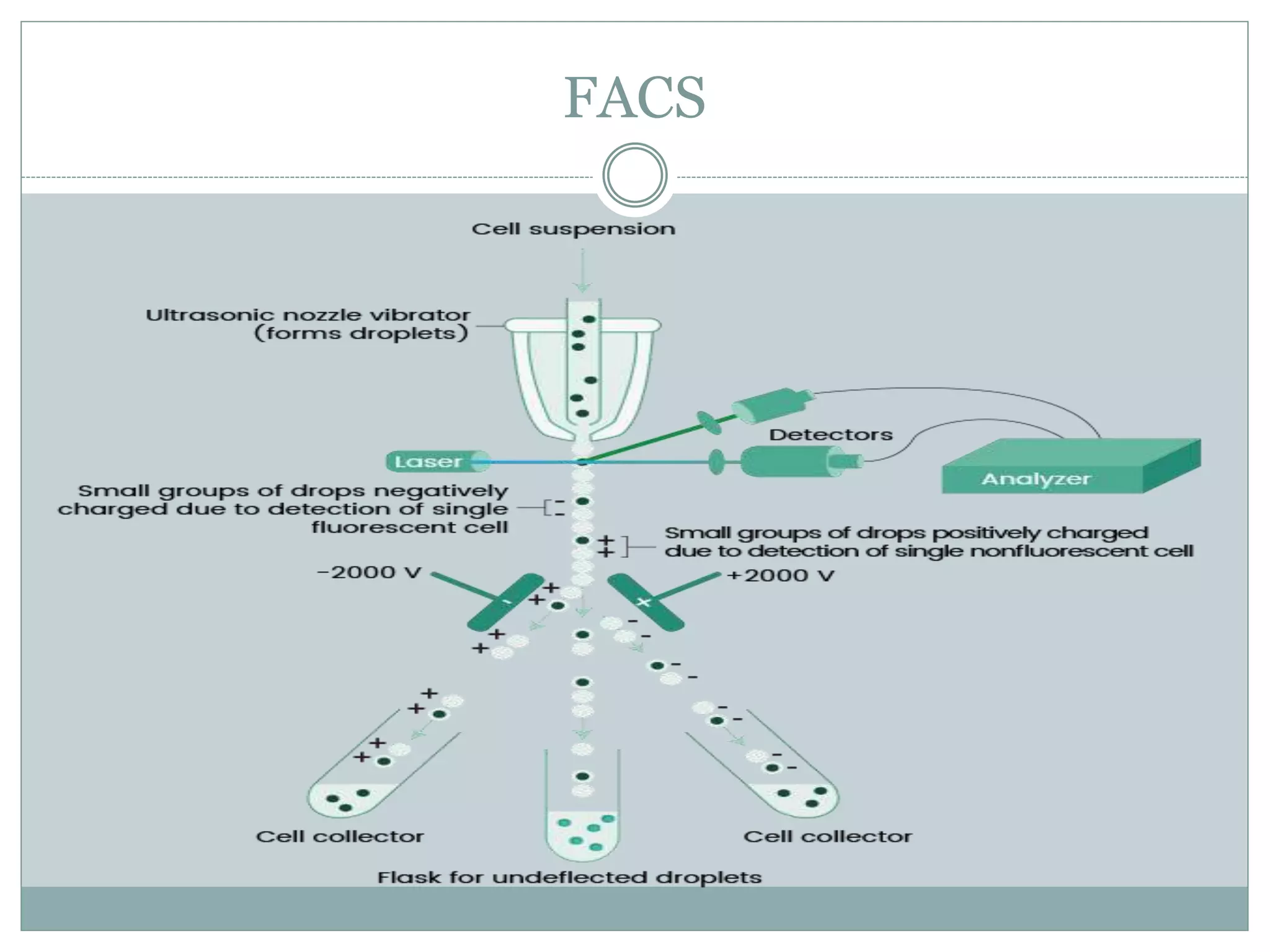
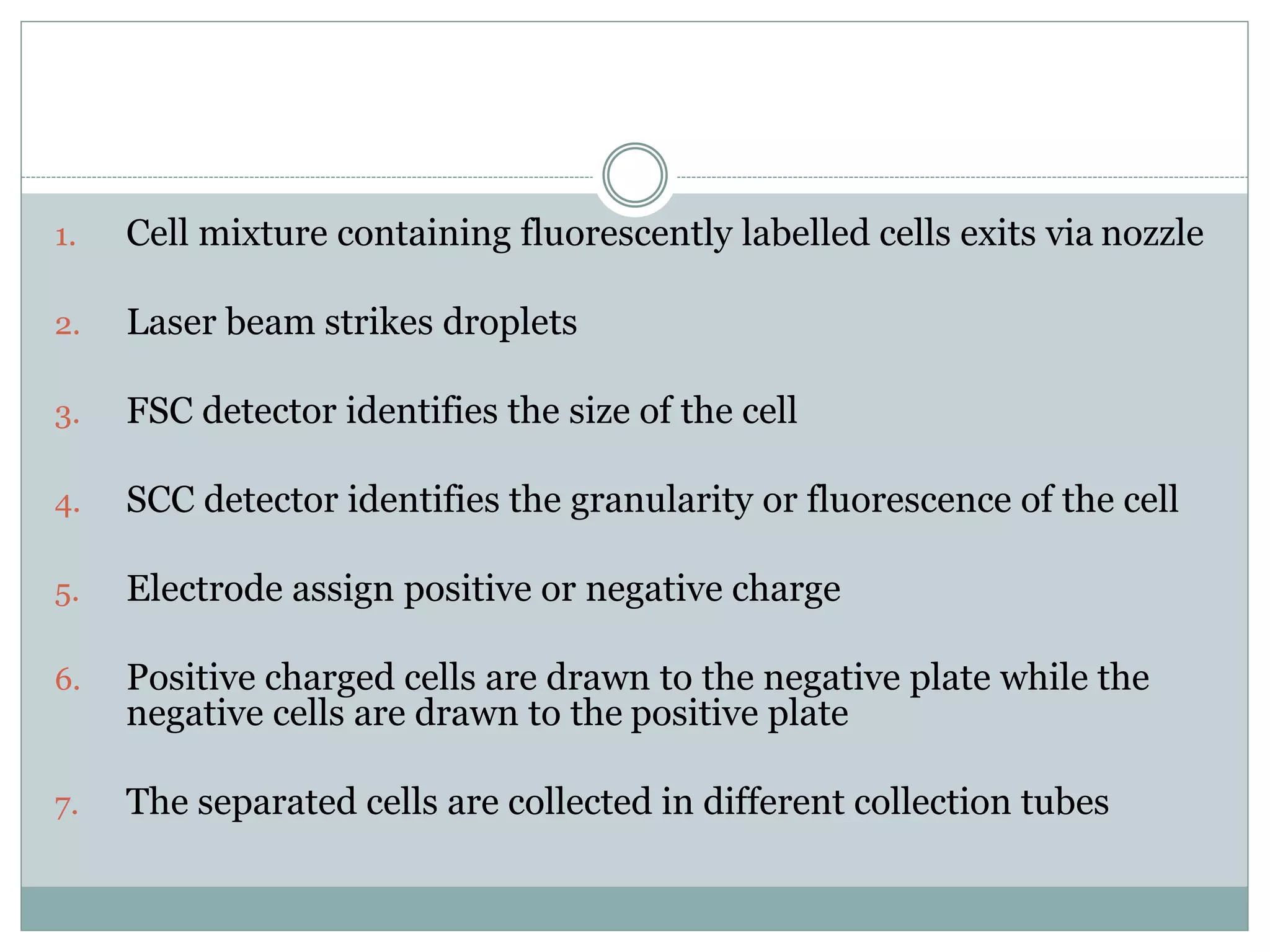
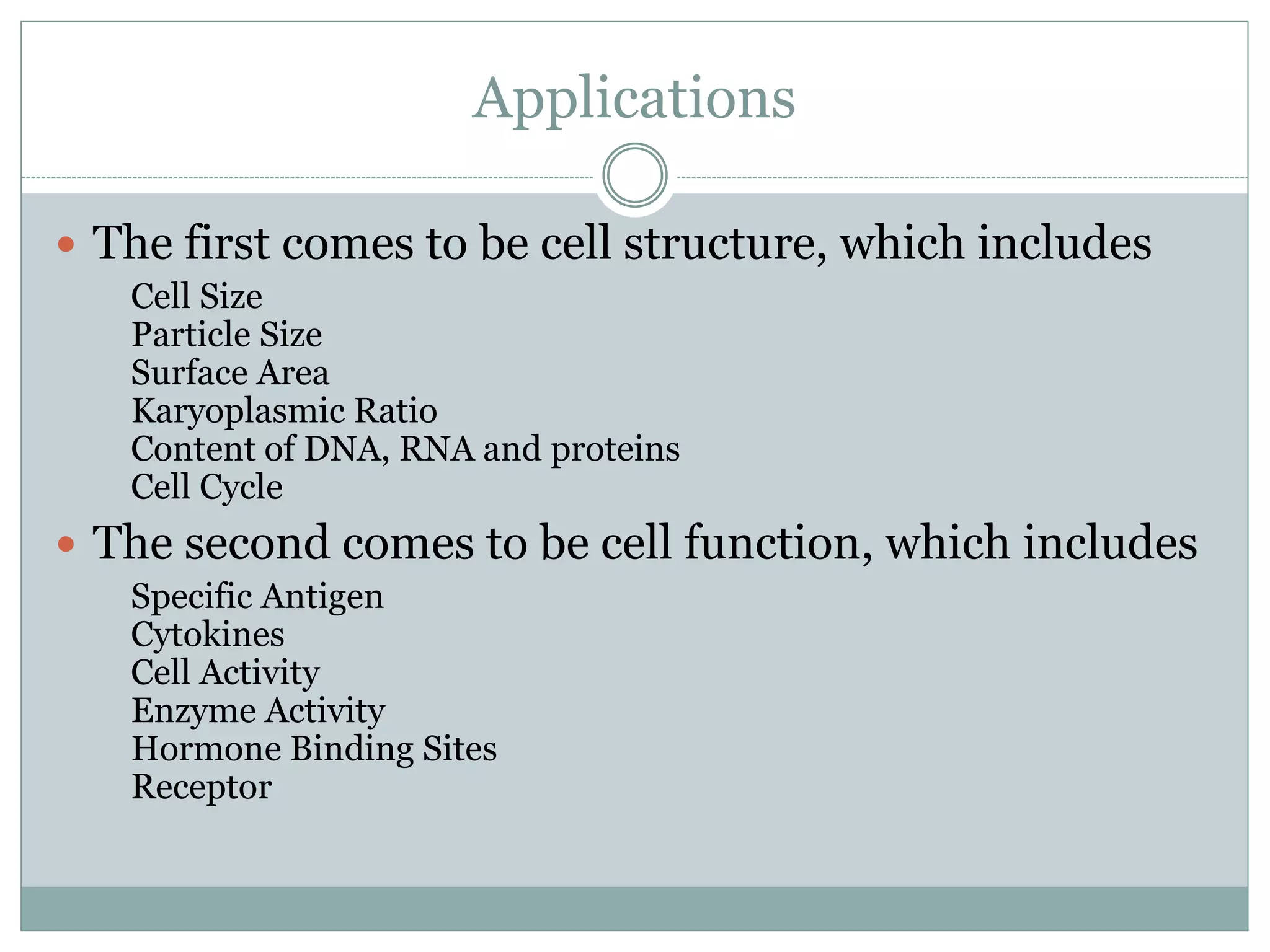
Fluorescence-activated cell sorting (FACS) is a specialized type of flow cytometry that allows for the sorting of cells into containers one by one based on their light scattering and fluorescent characteristics. FACS works by labeling cells with fluorescent markers and passing them through a laser beam, where detectors measure the light scattering and fluorescence to identify cell properties. This technique allows researchers to isolate targeted cell groups for further analysis and is commonly used in medical research areas like hematology and oncology.