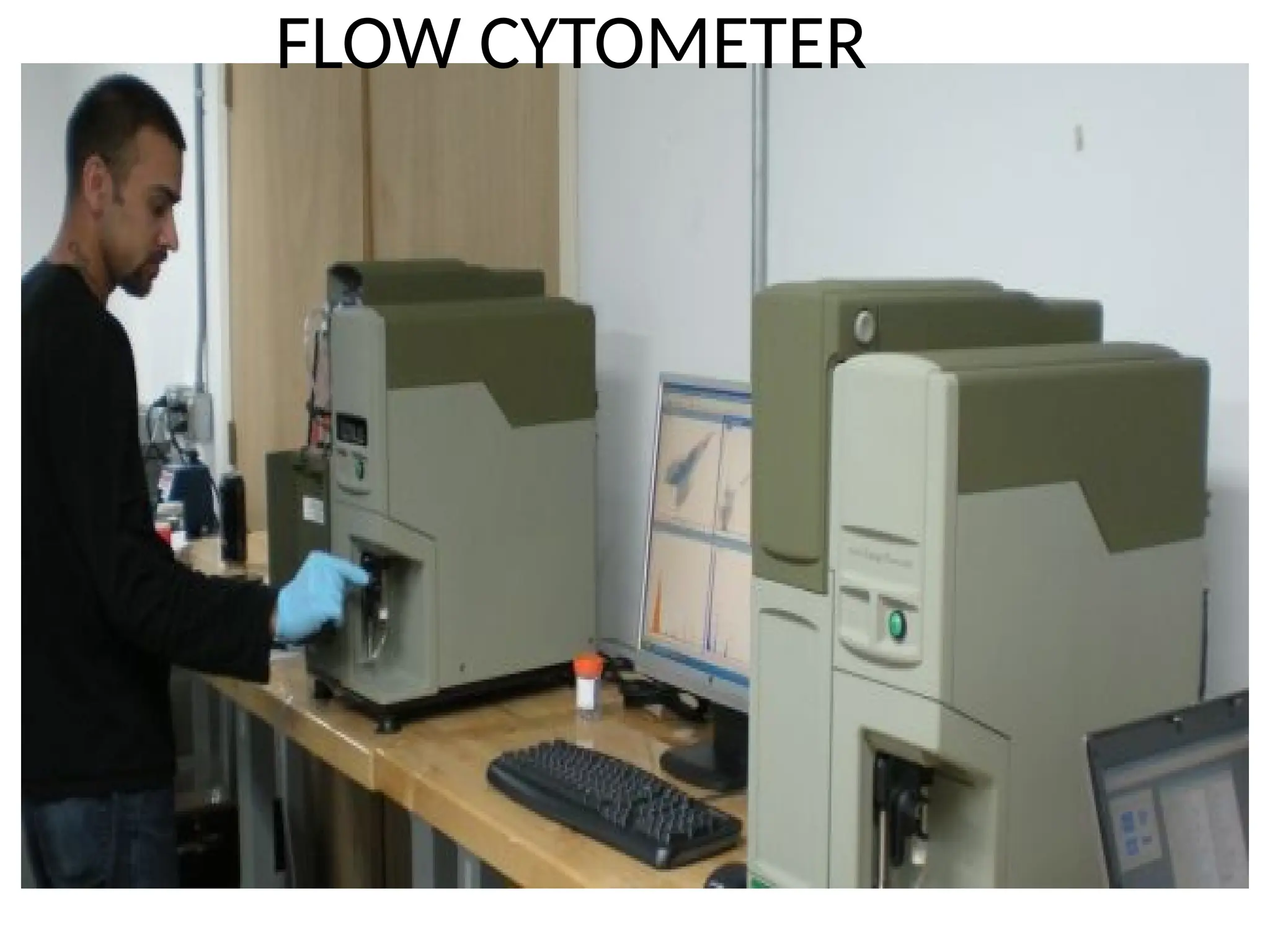
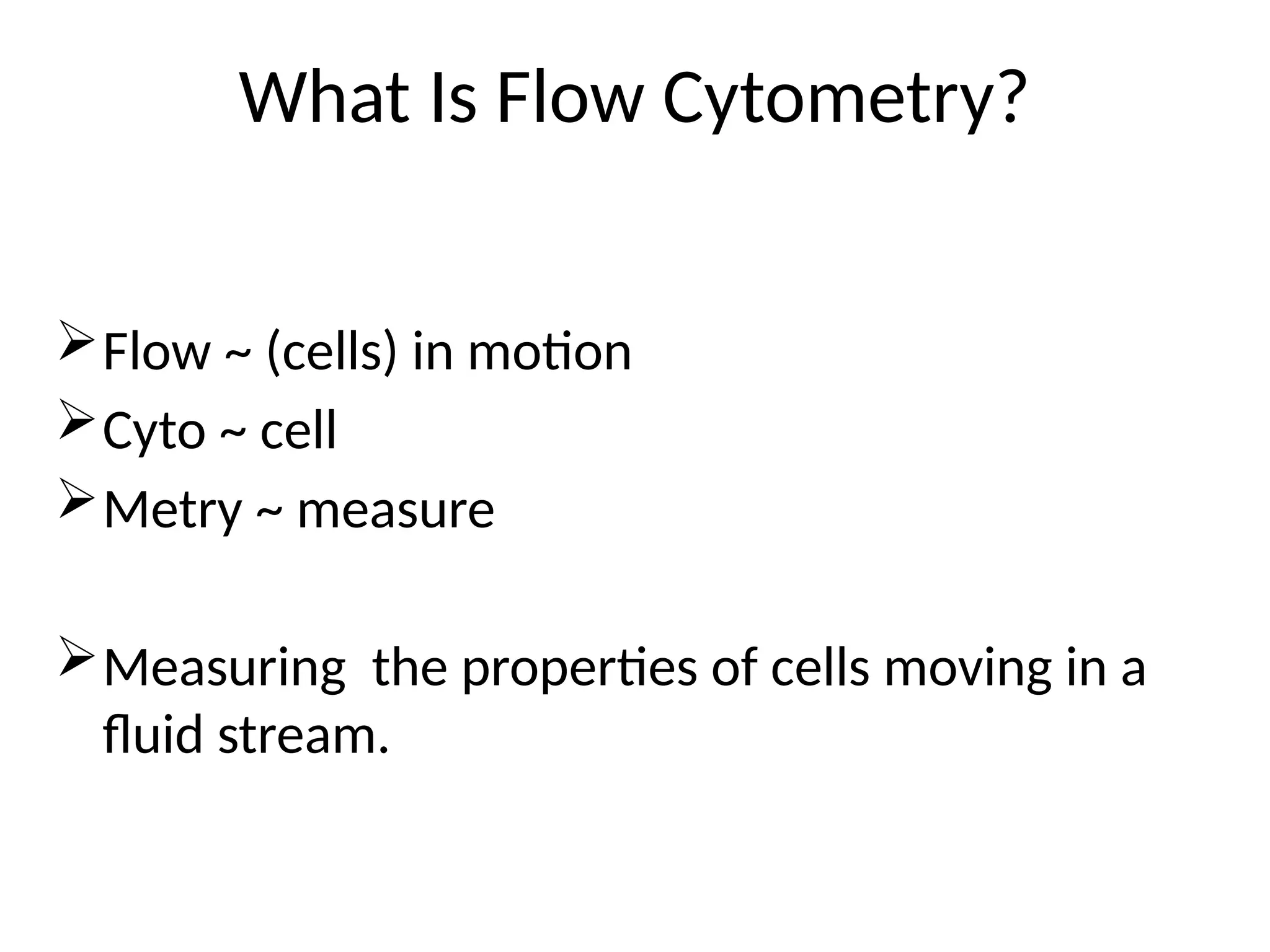
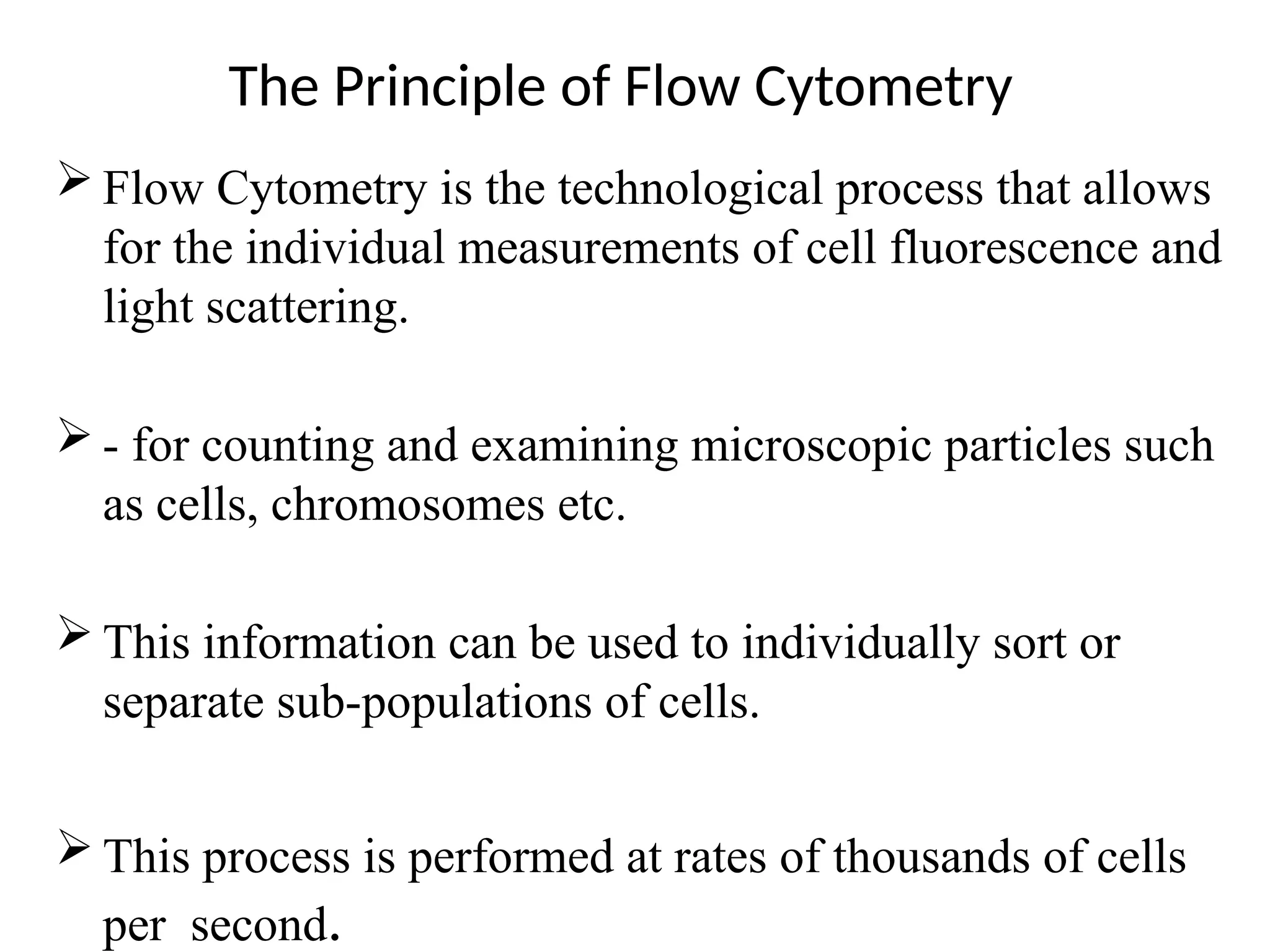
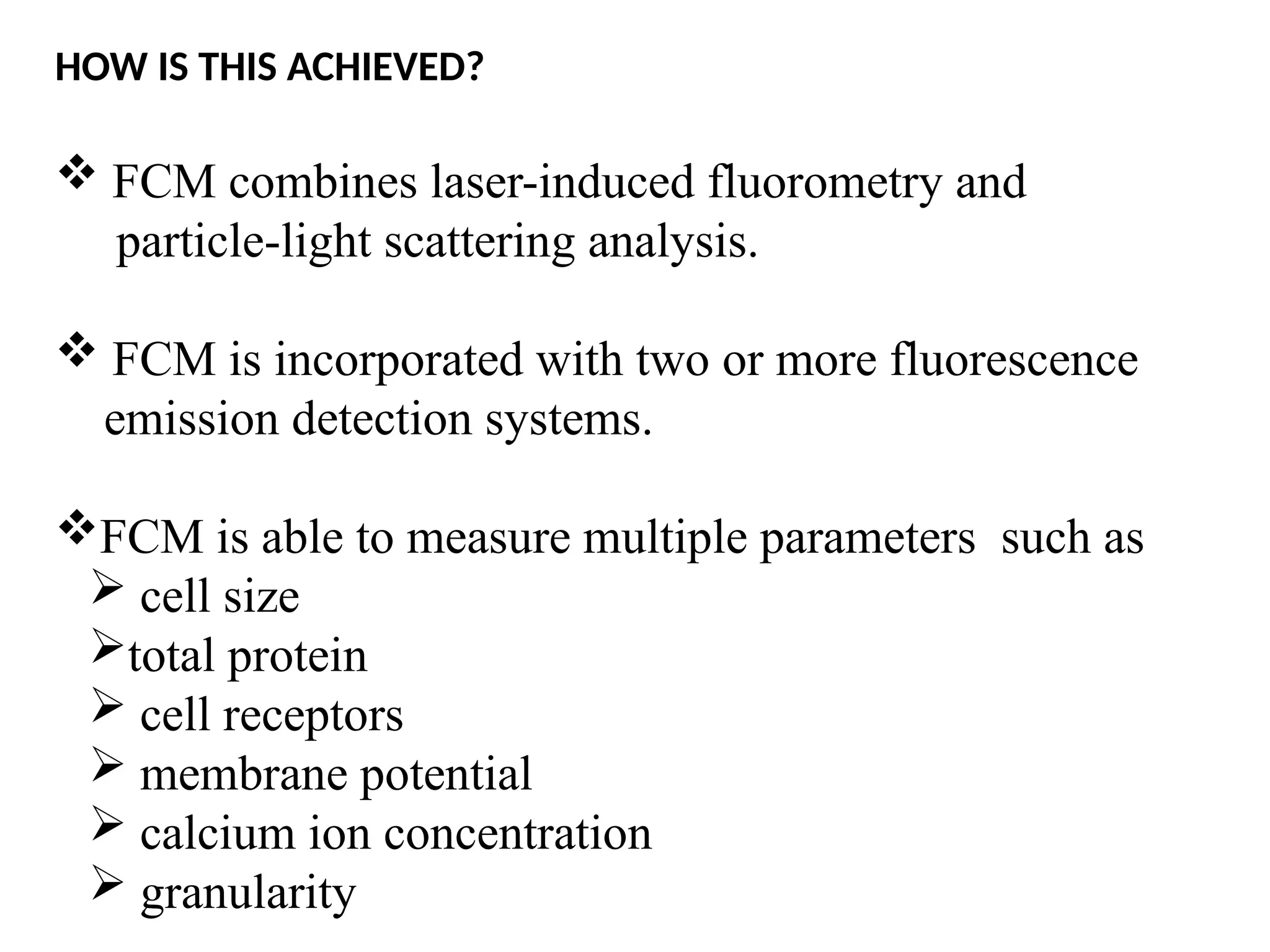
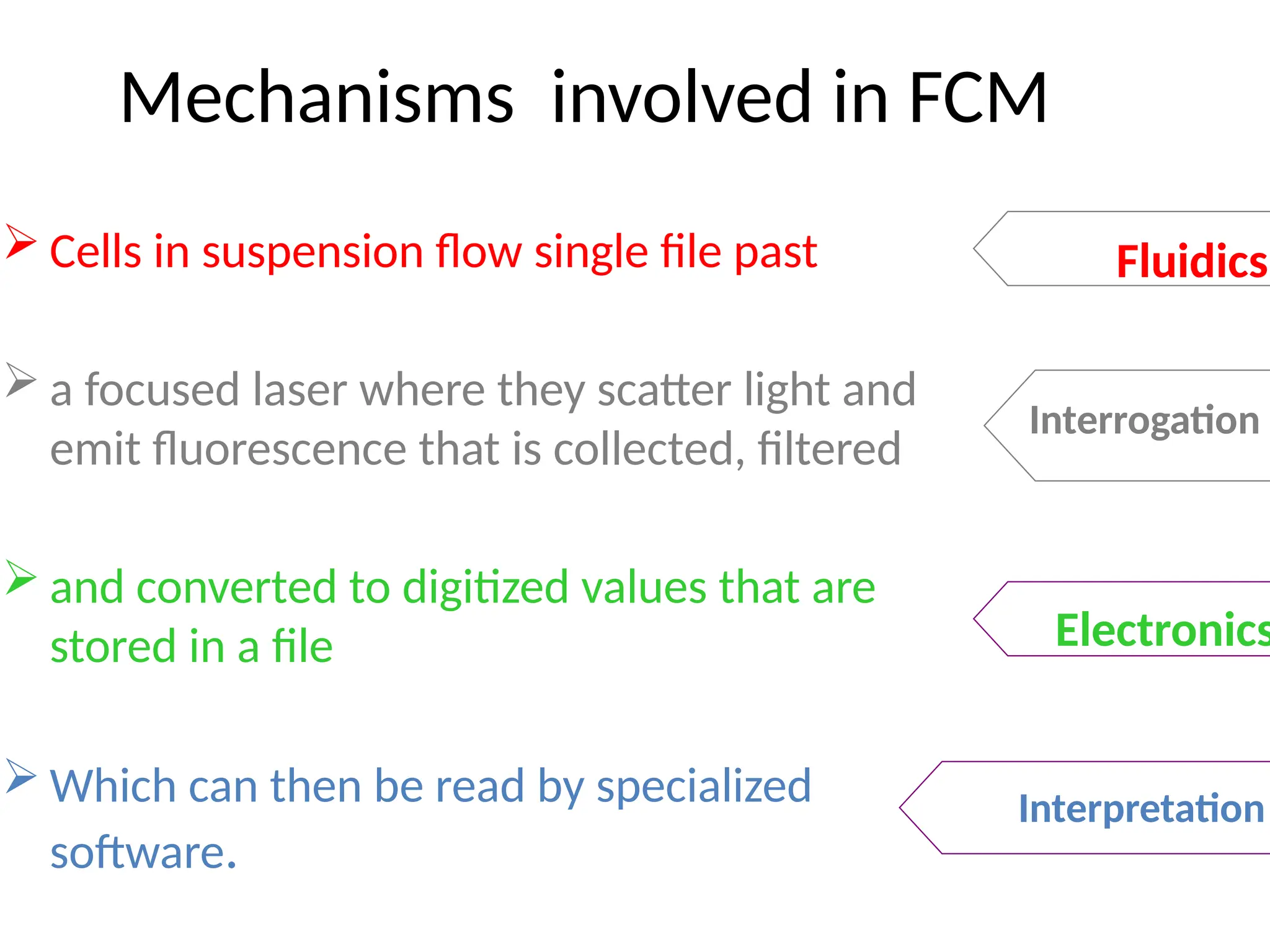
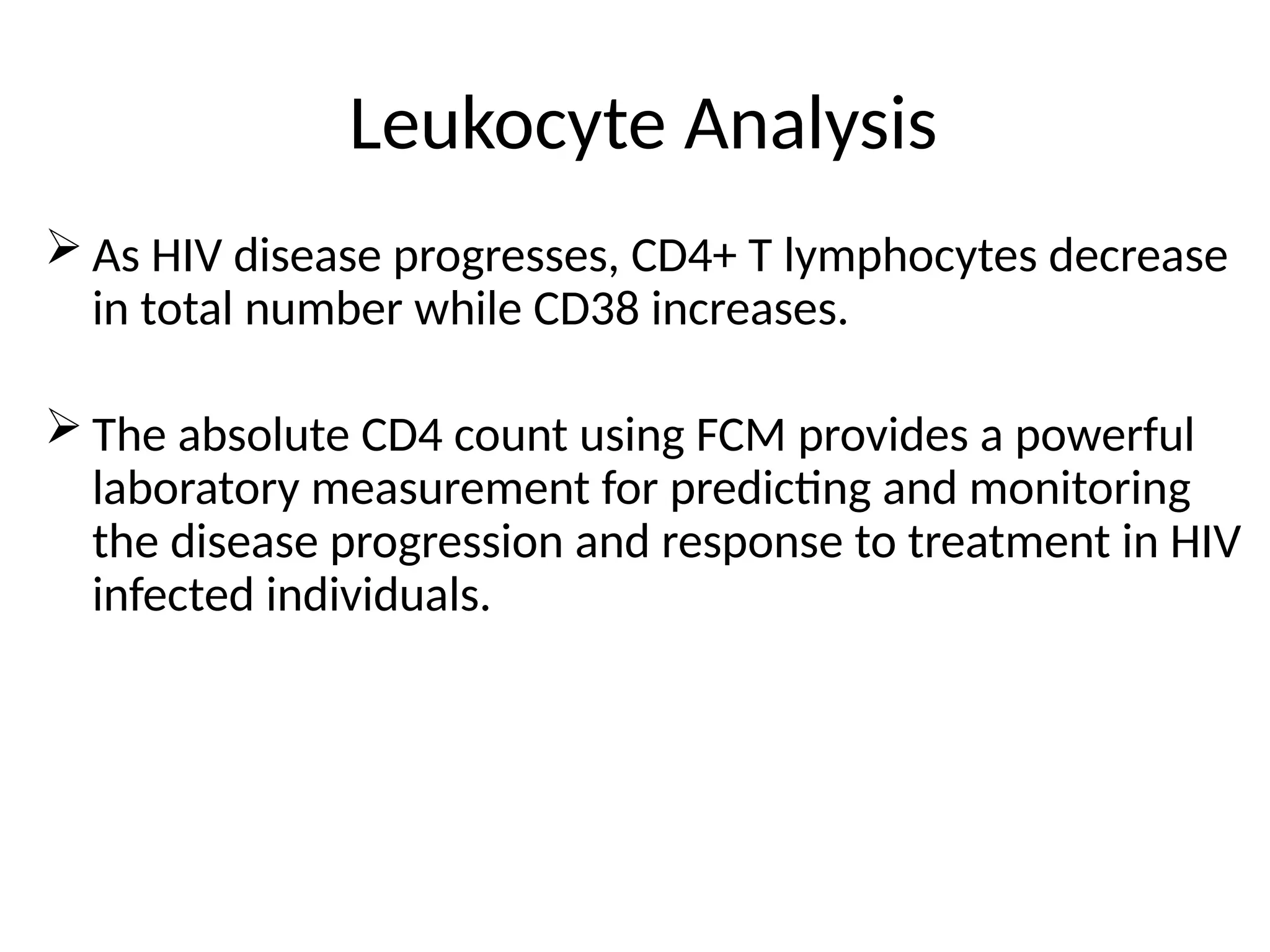
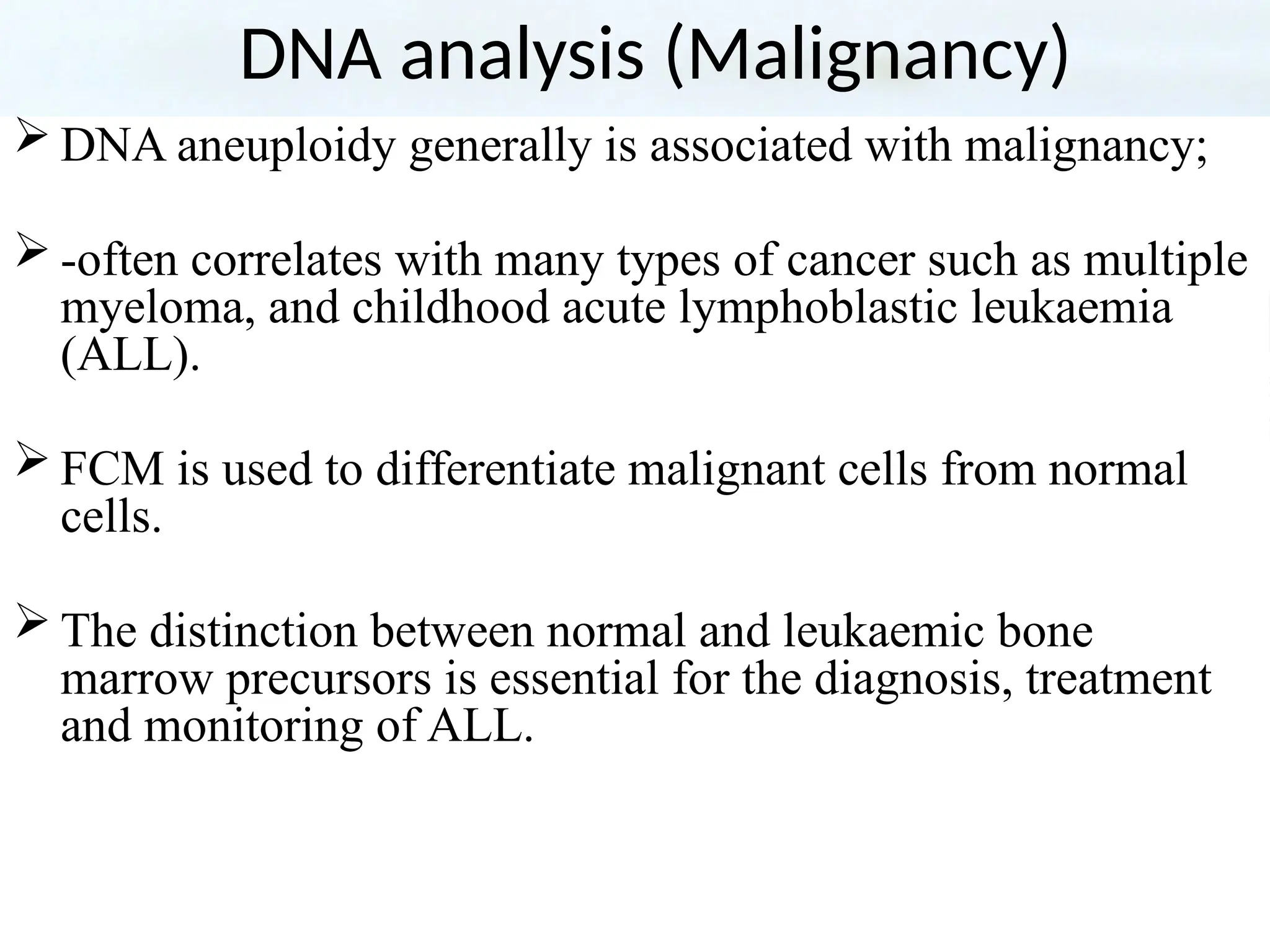
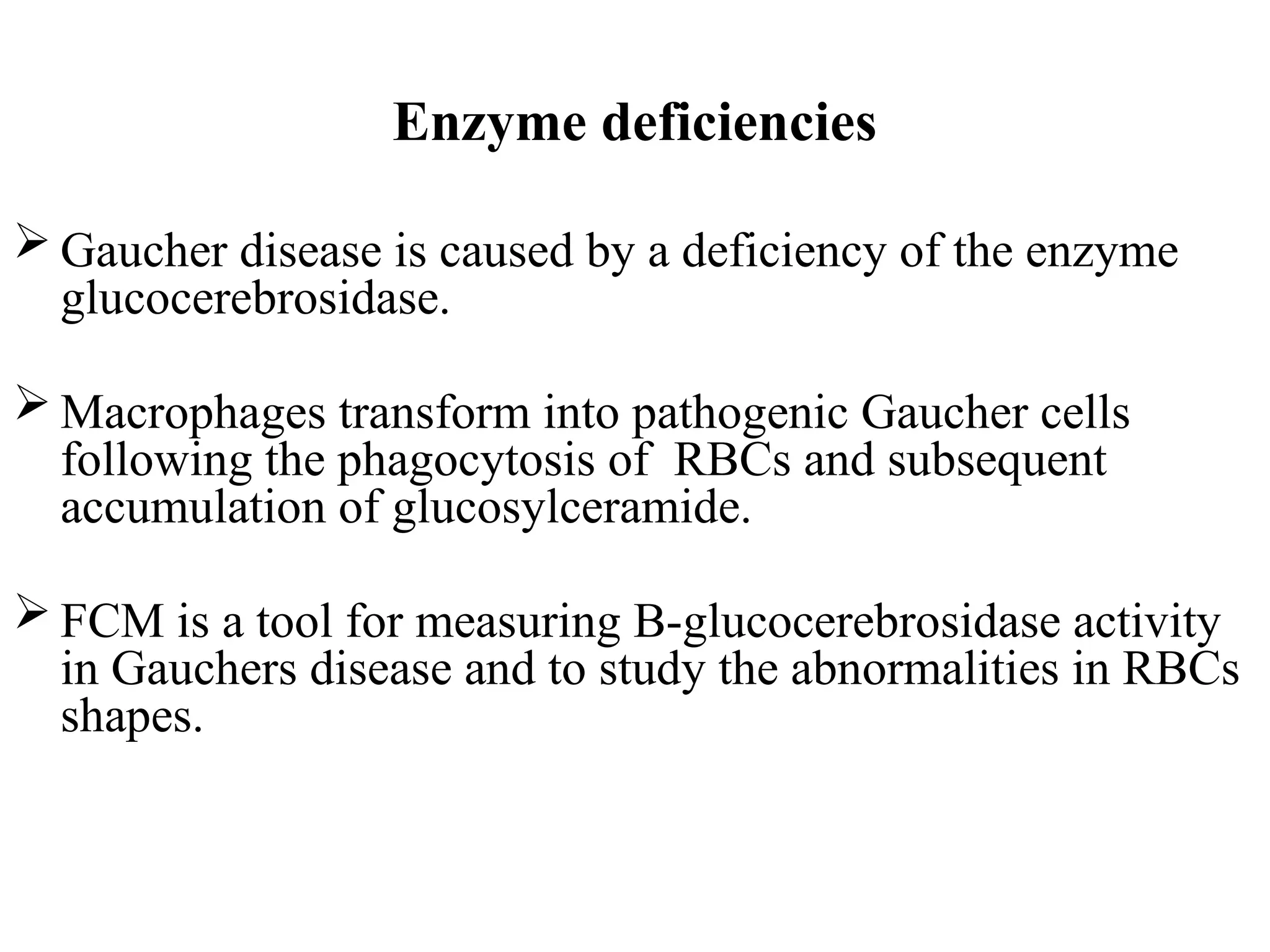
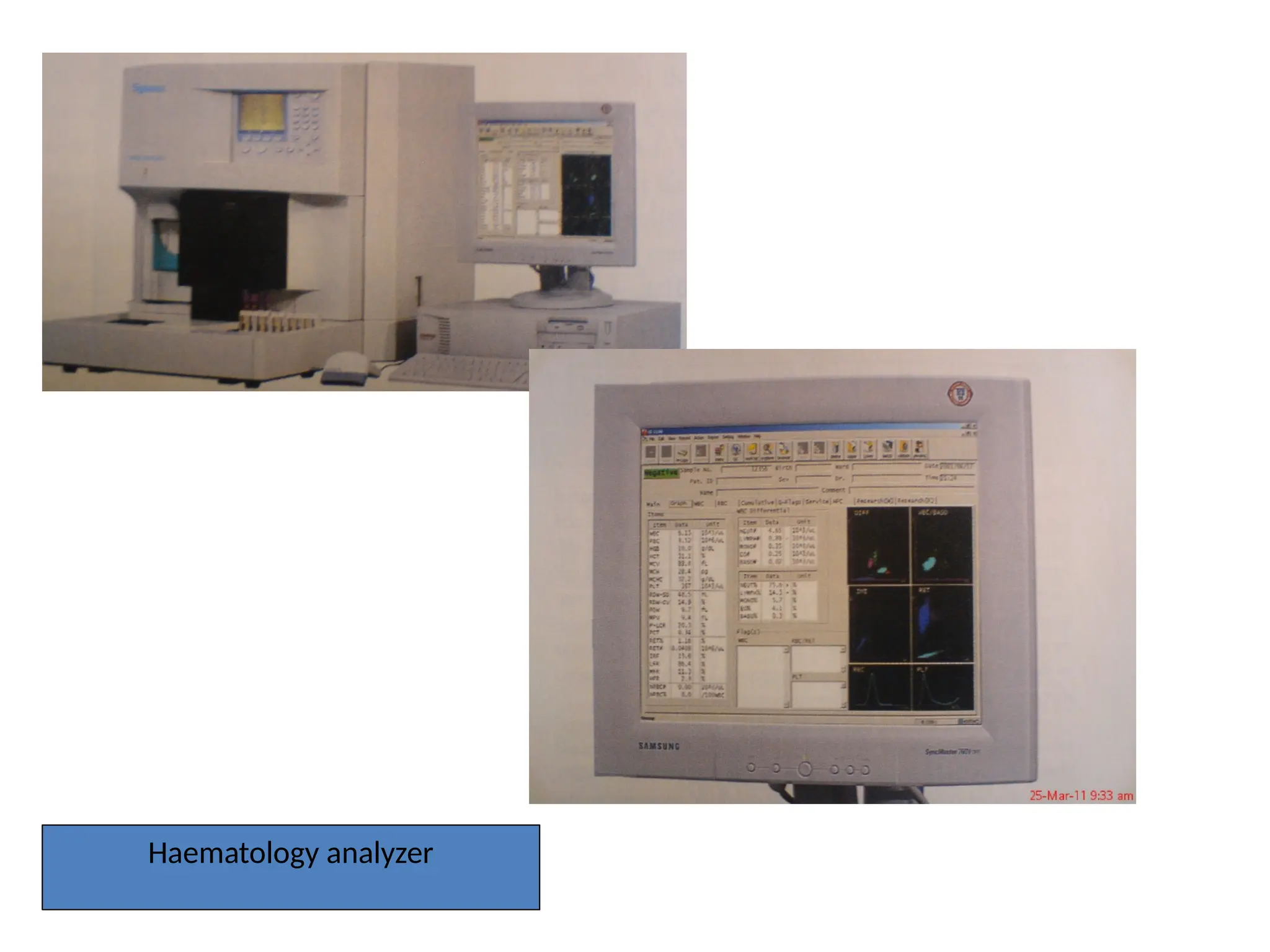
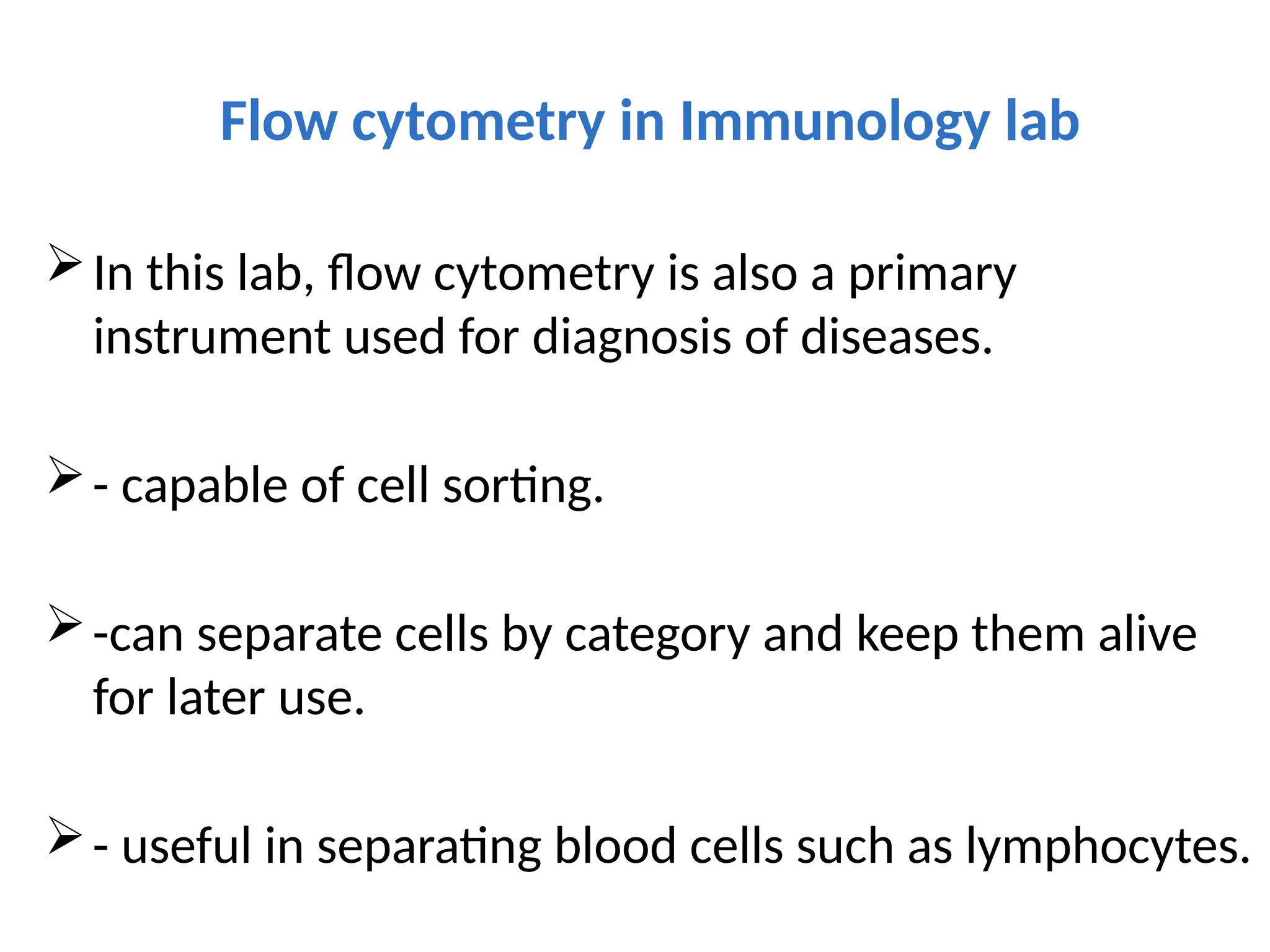
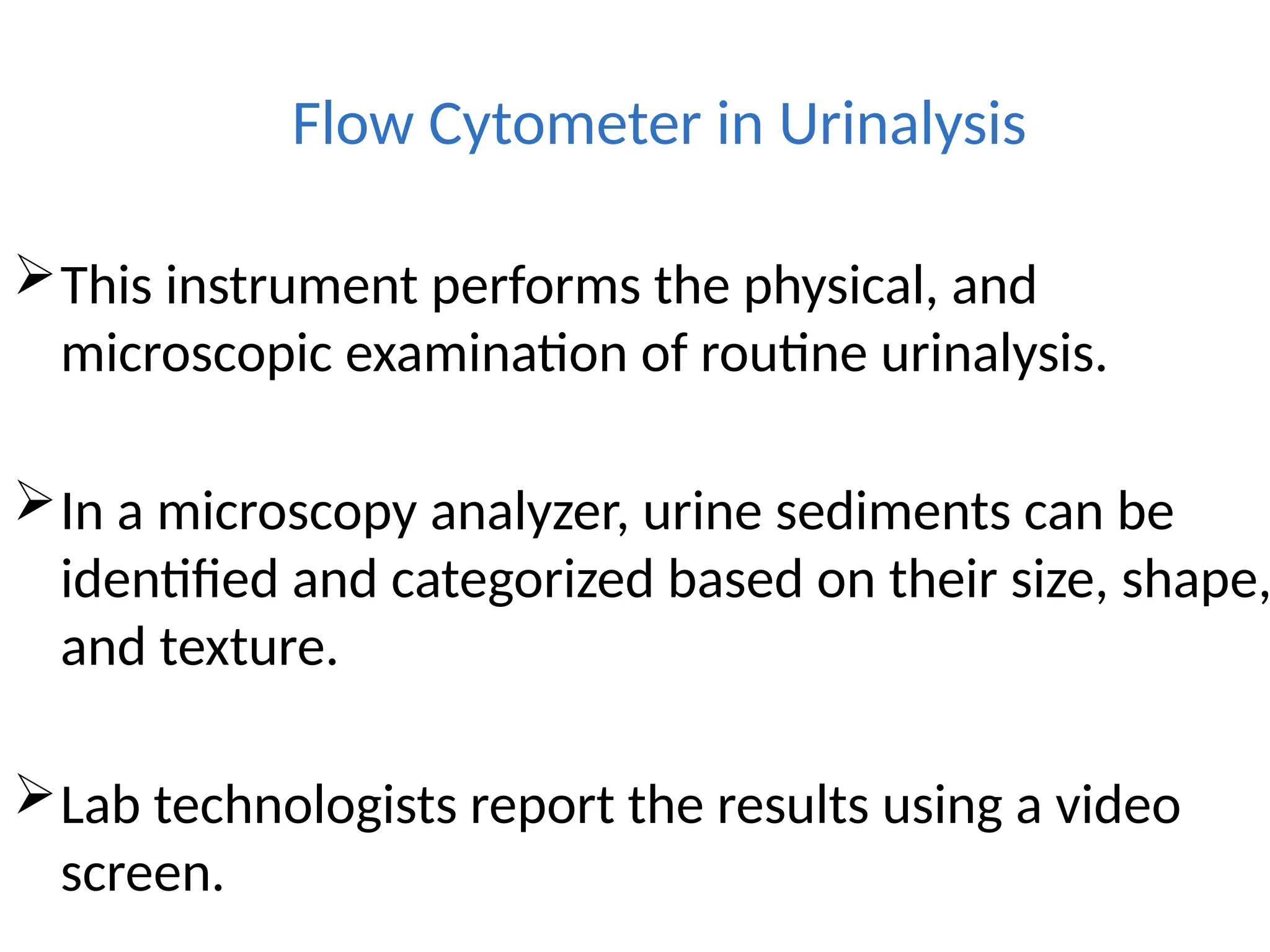
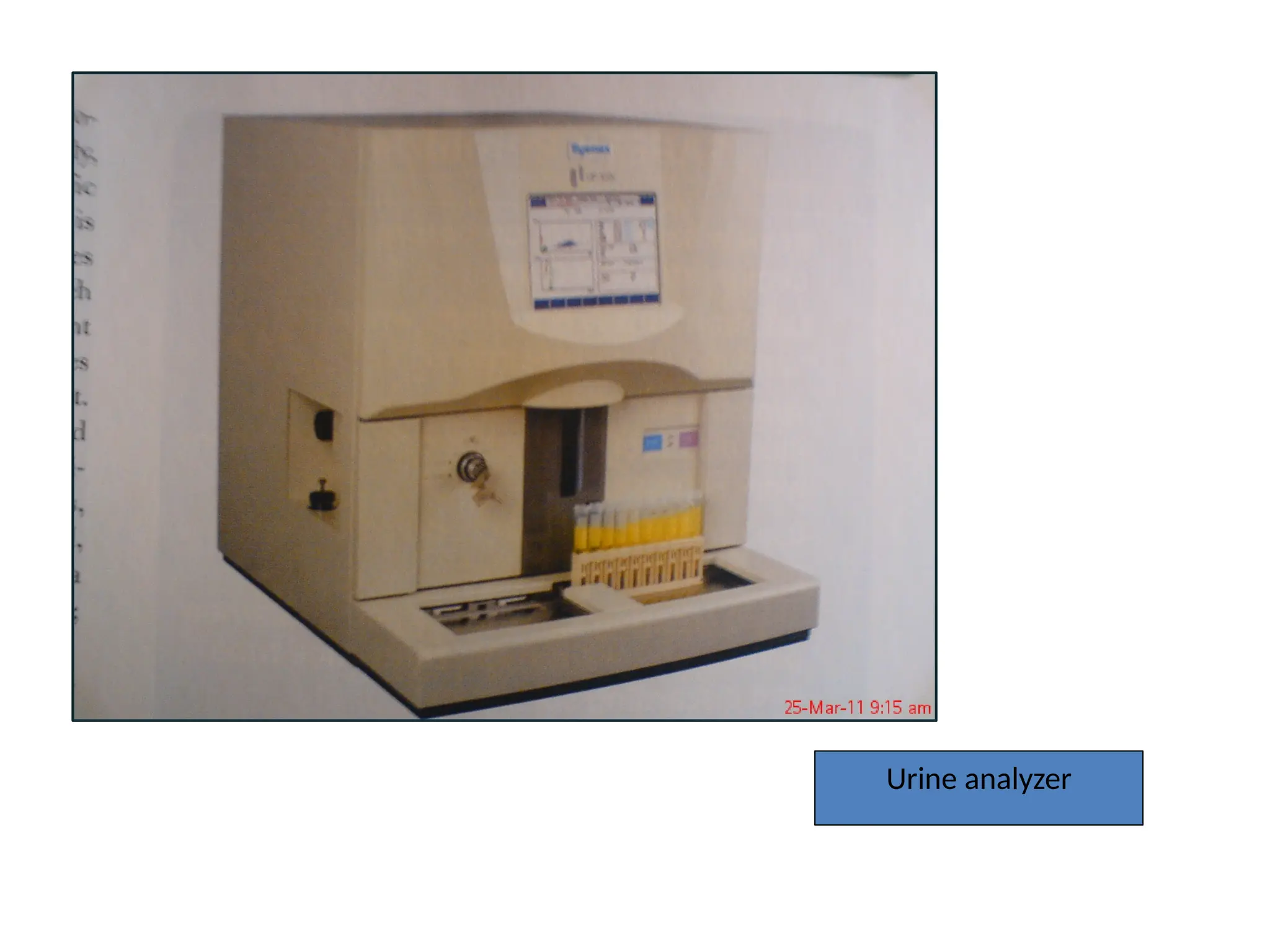
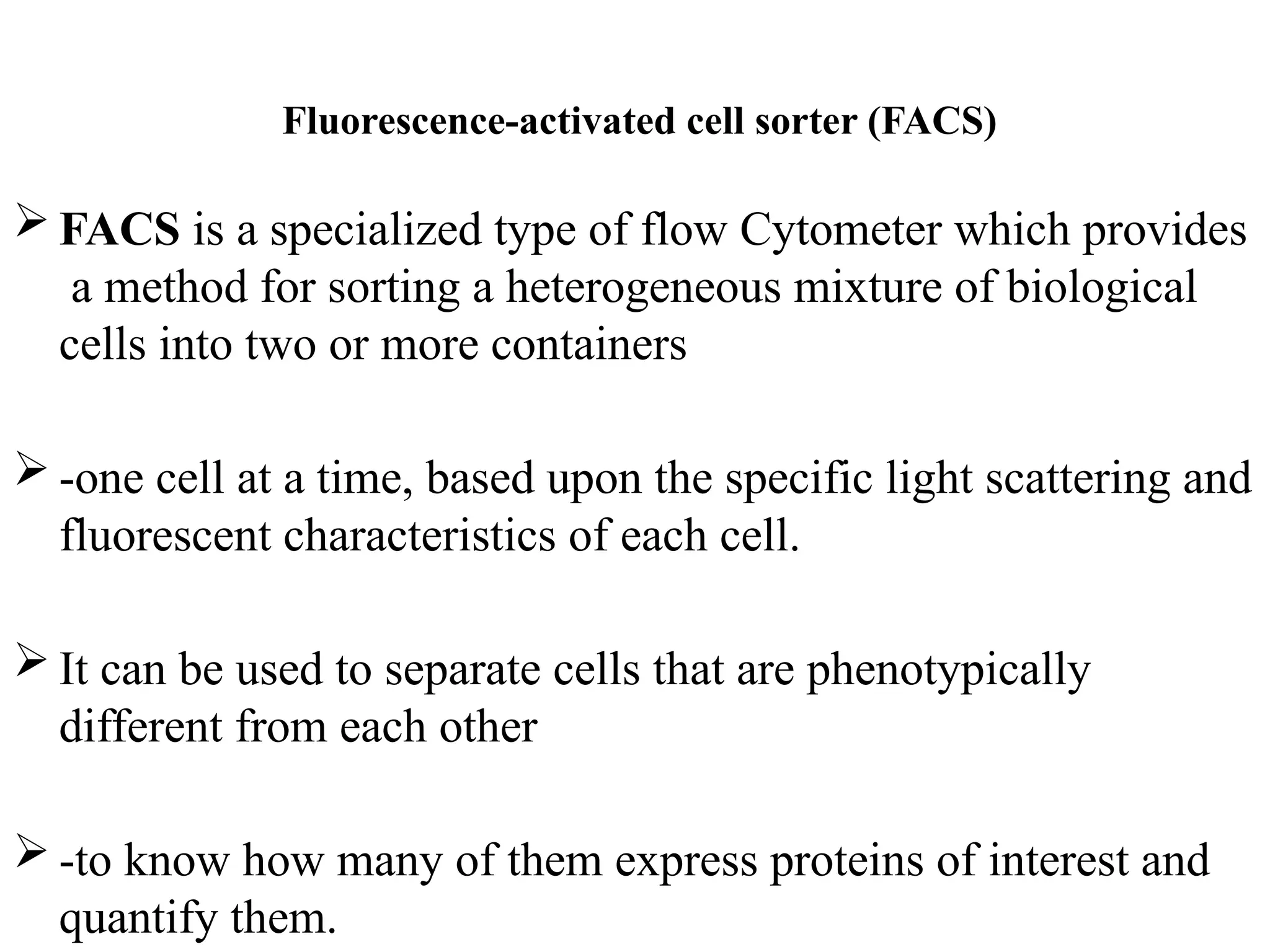
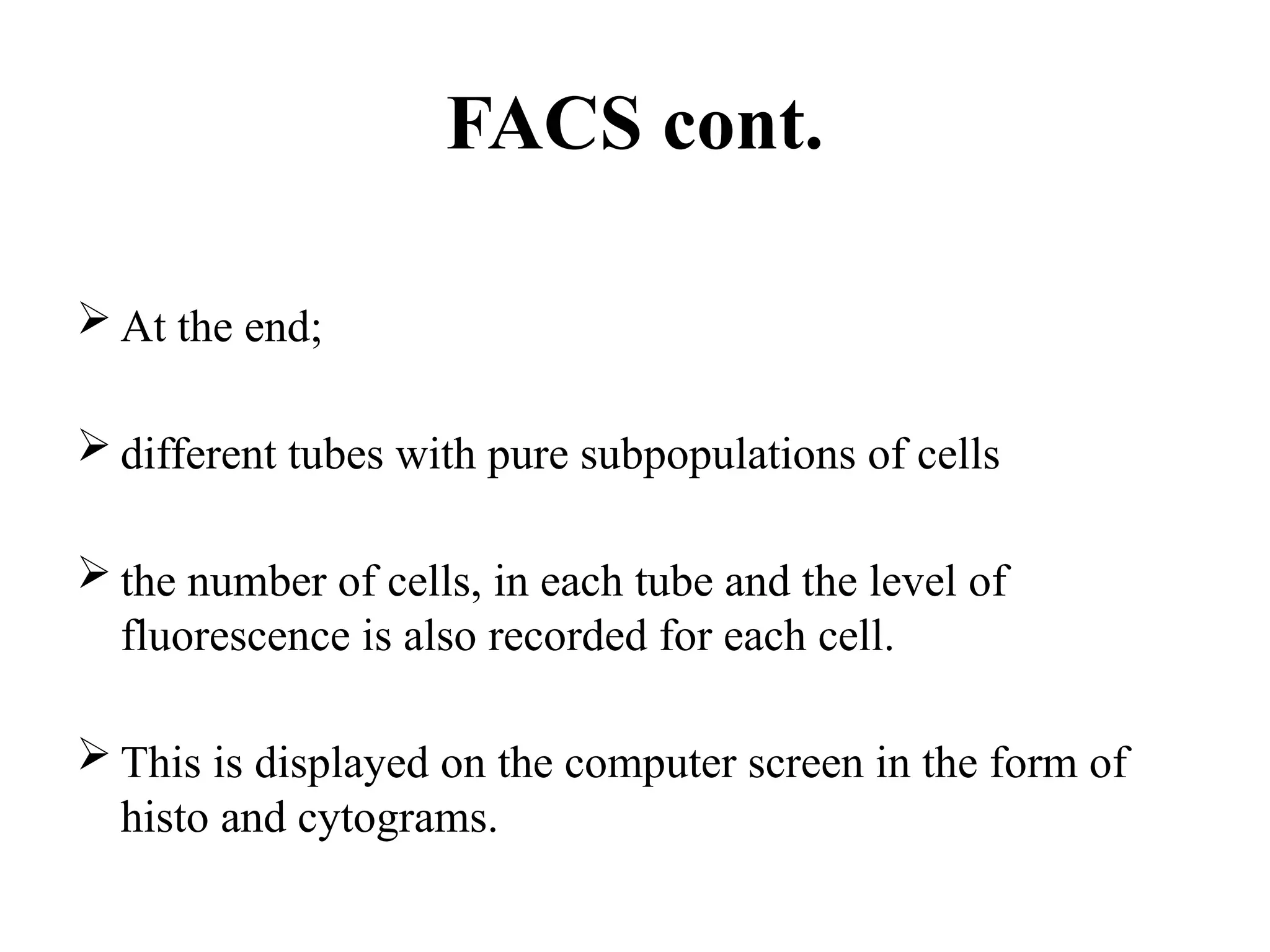
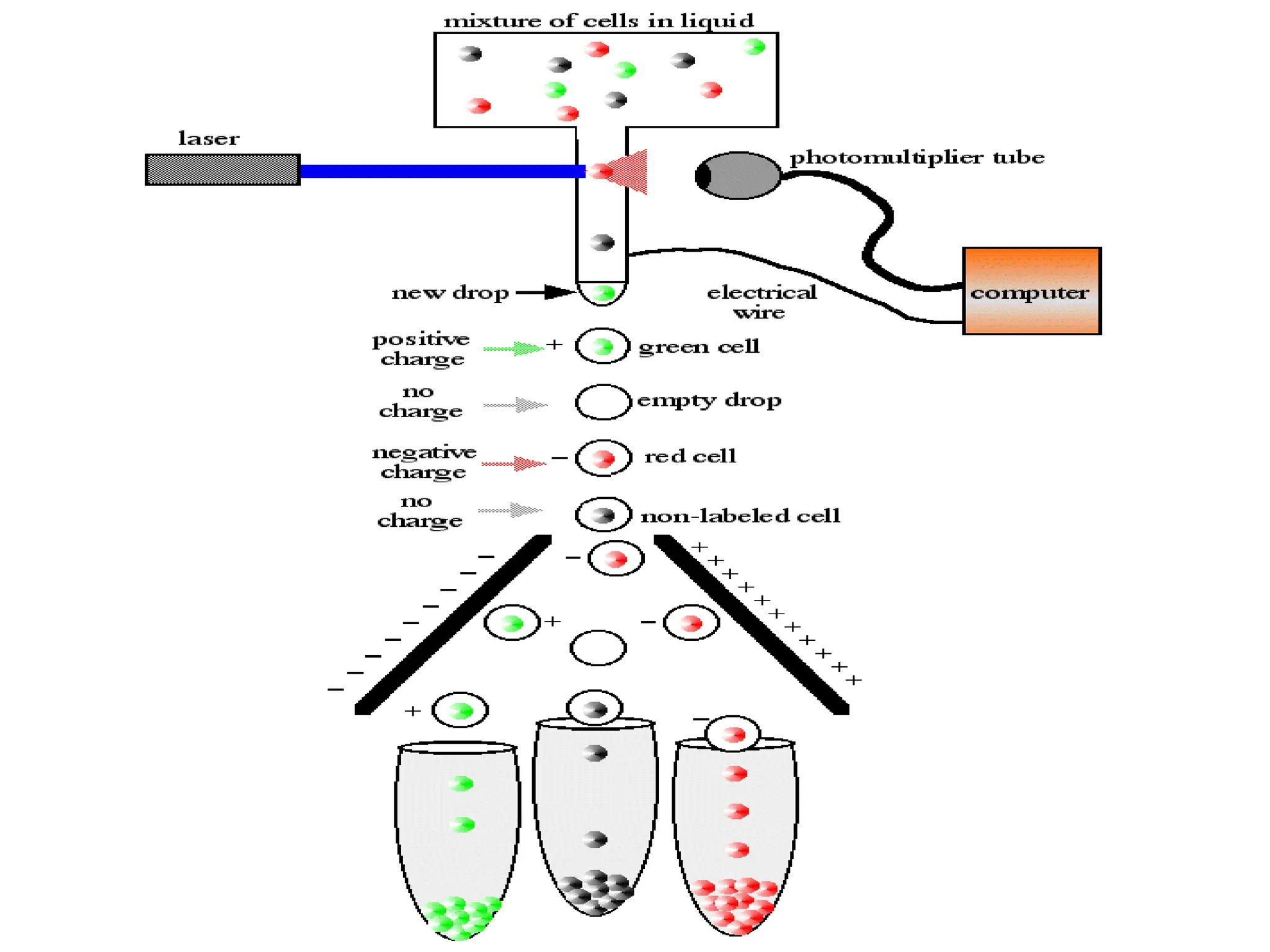
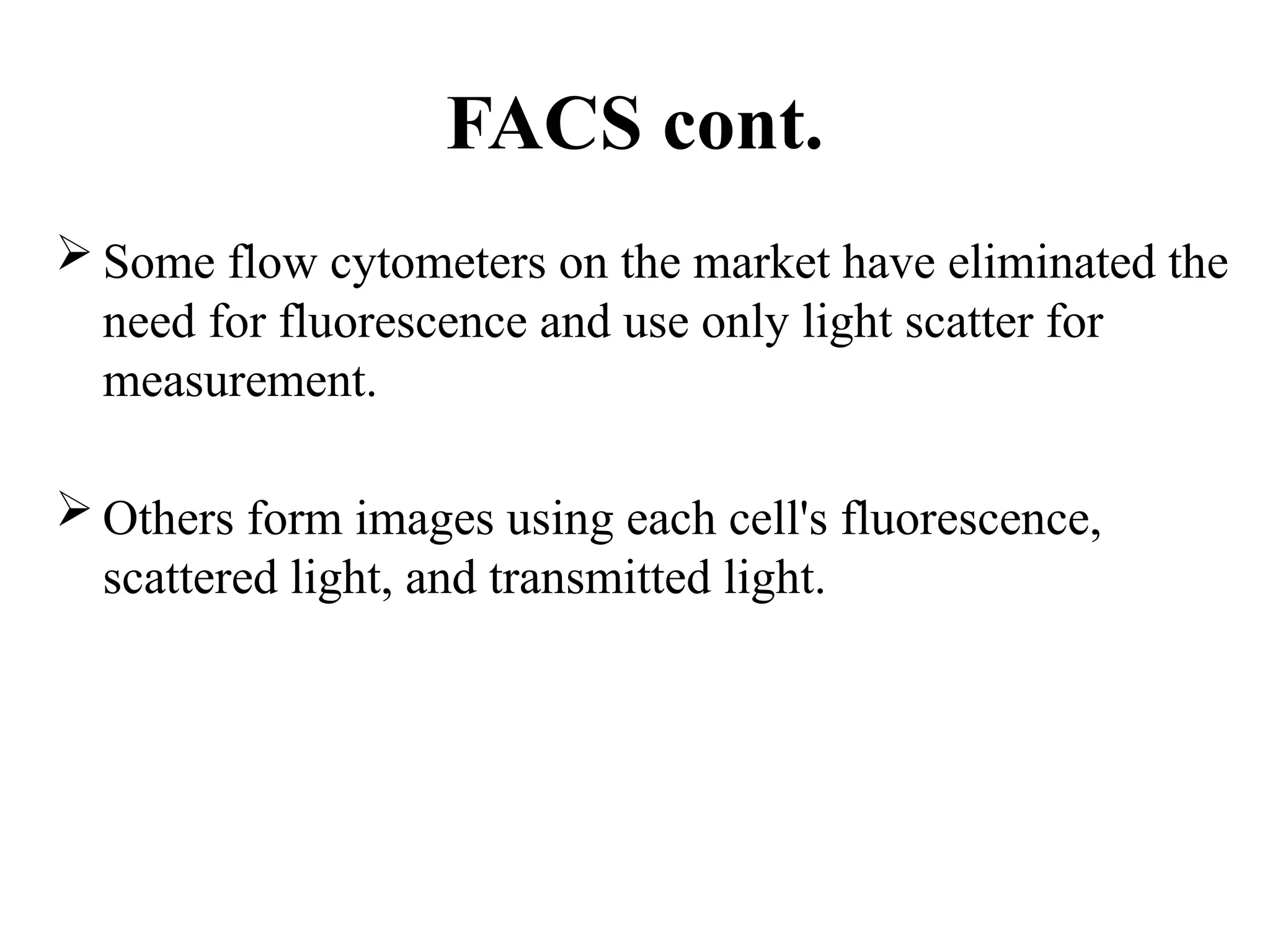
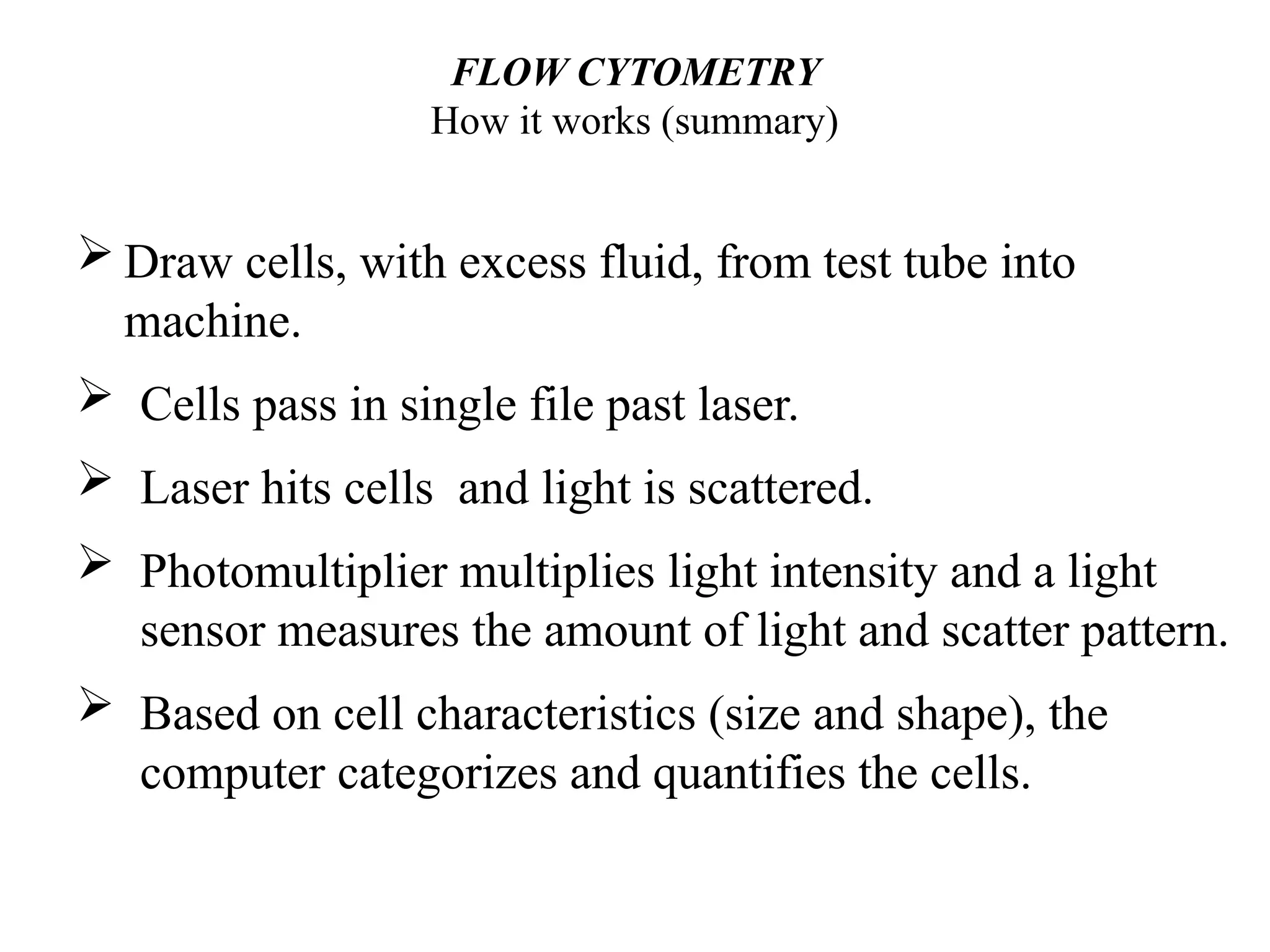
The document provides a comprehensive overview of flow cytometry, detailing its principles, components, and applications in various medical laboratories for diagnosing diseases. It explains how the technology measures cell properties by analyzing light scattering and fluorescence, allowing for detailed cellular assessments and sorting. Flow cytometry is highlighted as a crucial tool for analyzing blood cells, immunophenotyping, and identifying genetic diseases.













![CHEMICALS
• 2.0% sodium metabisulphite
• 5.0% EDTA
• 3.2% Trisodium Citrate
• TEB BUFFER [electrophoretic buffer (pH 8.2-8.6)]
• TRIS- 10.2g
• EDTA-0.6g
• BORIC ACID- 3.2g](https://image.slidesharecdn.com/l200lectureflowcytometry-241002115621-1fc6f39f/75/Flow-Cytometry-A-widely-used-mechanism-in-automated-instruments-pptx-48-2048.jpg)