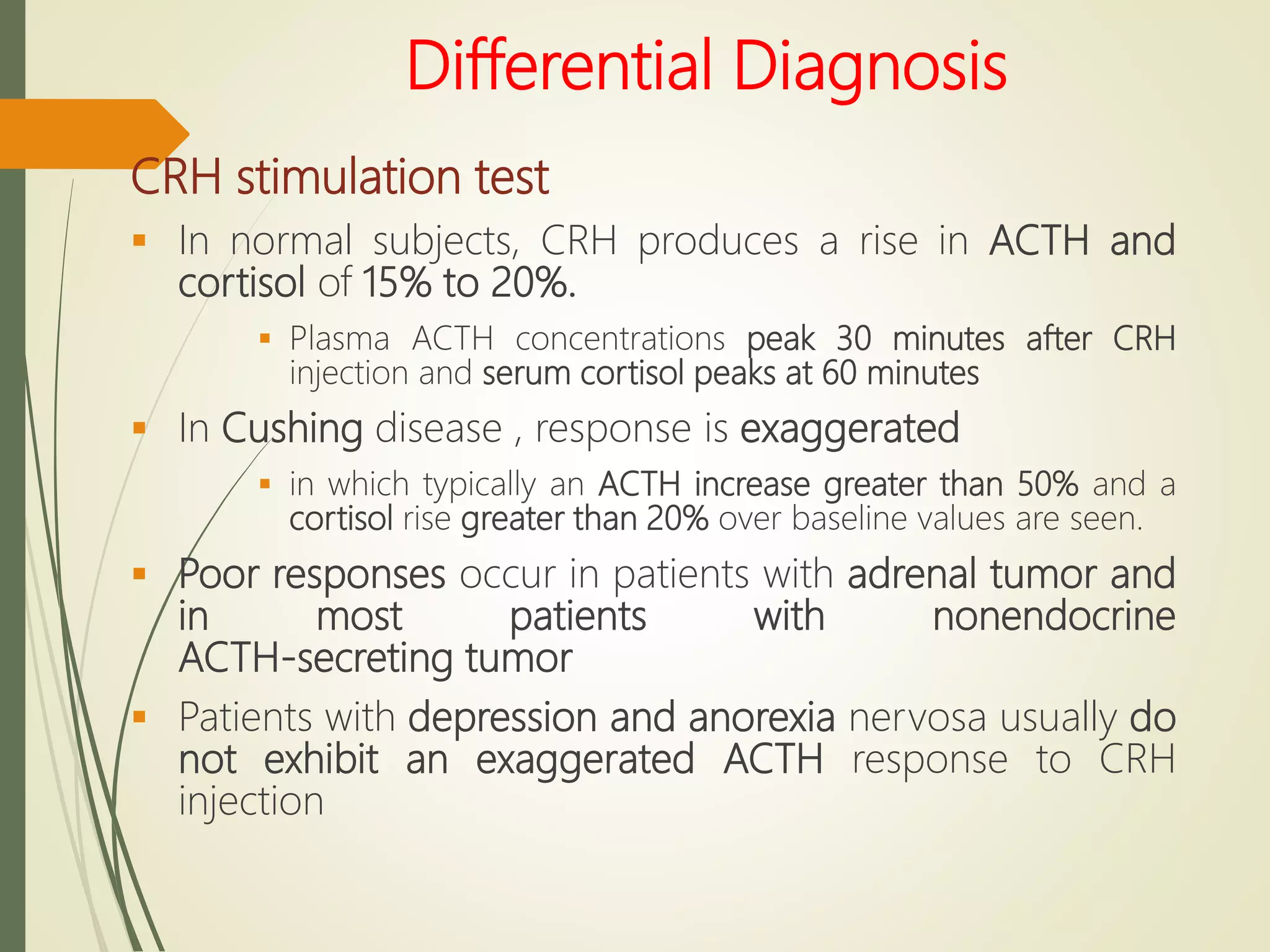
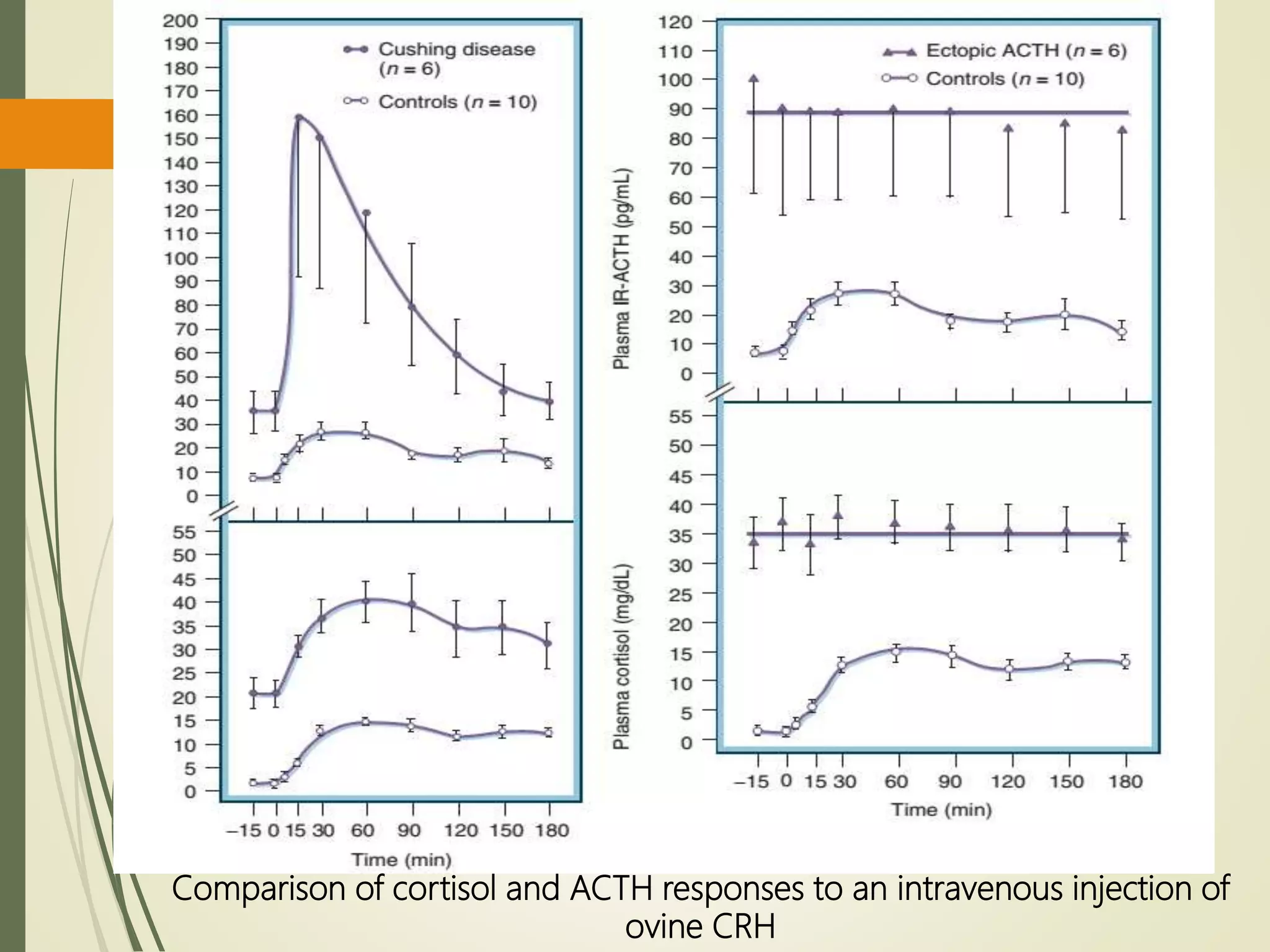
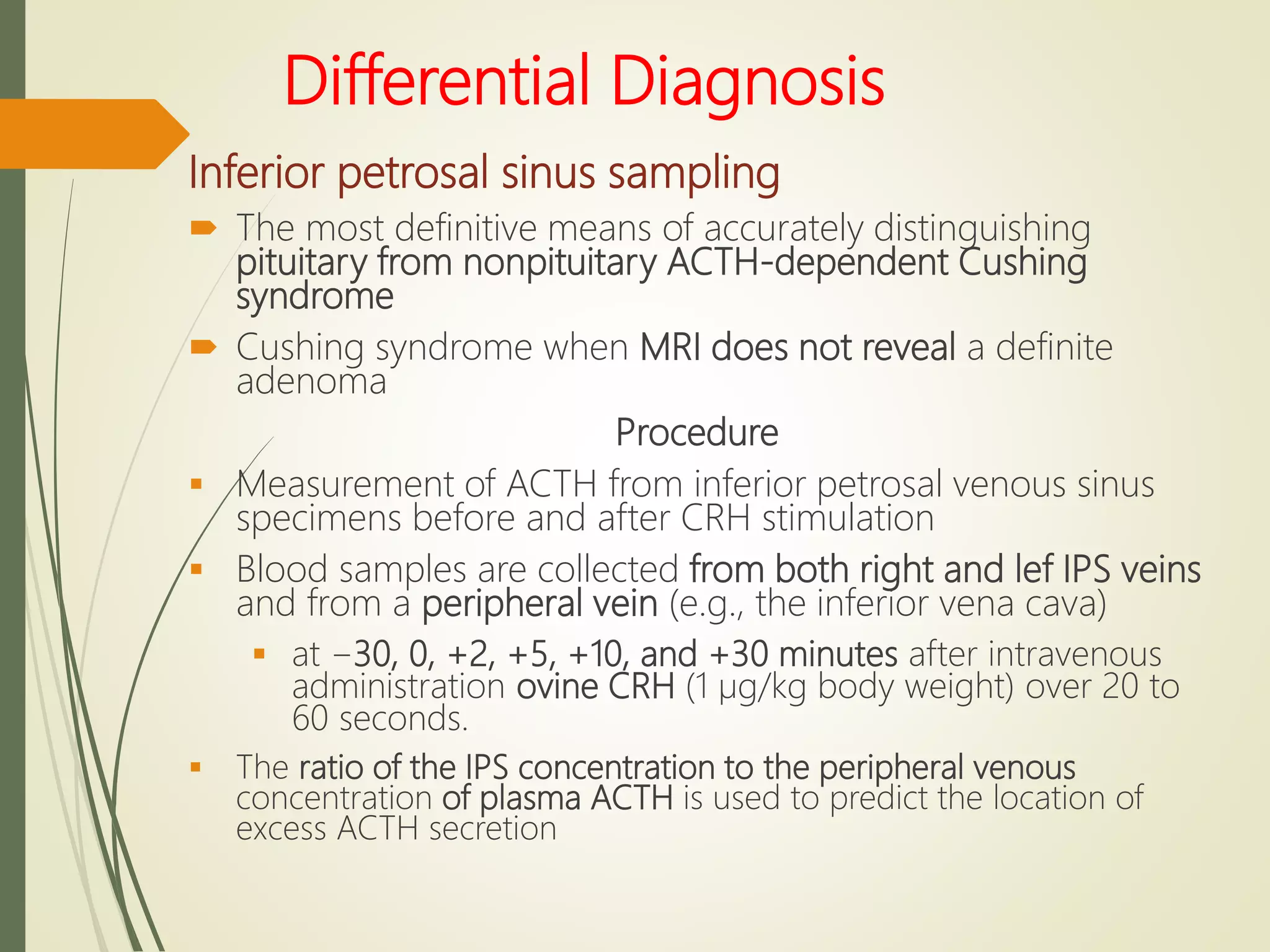
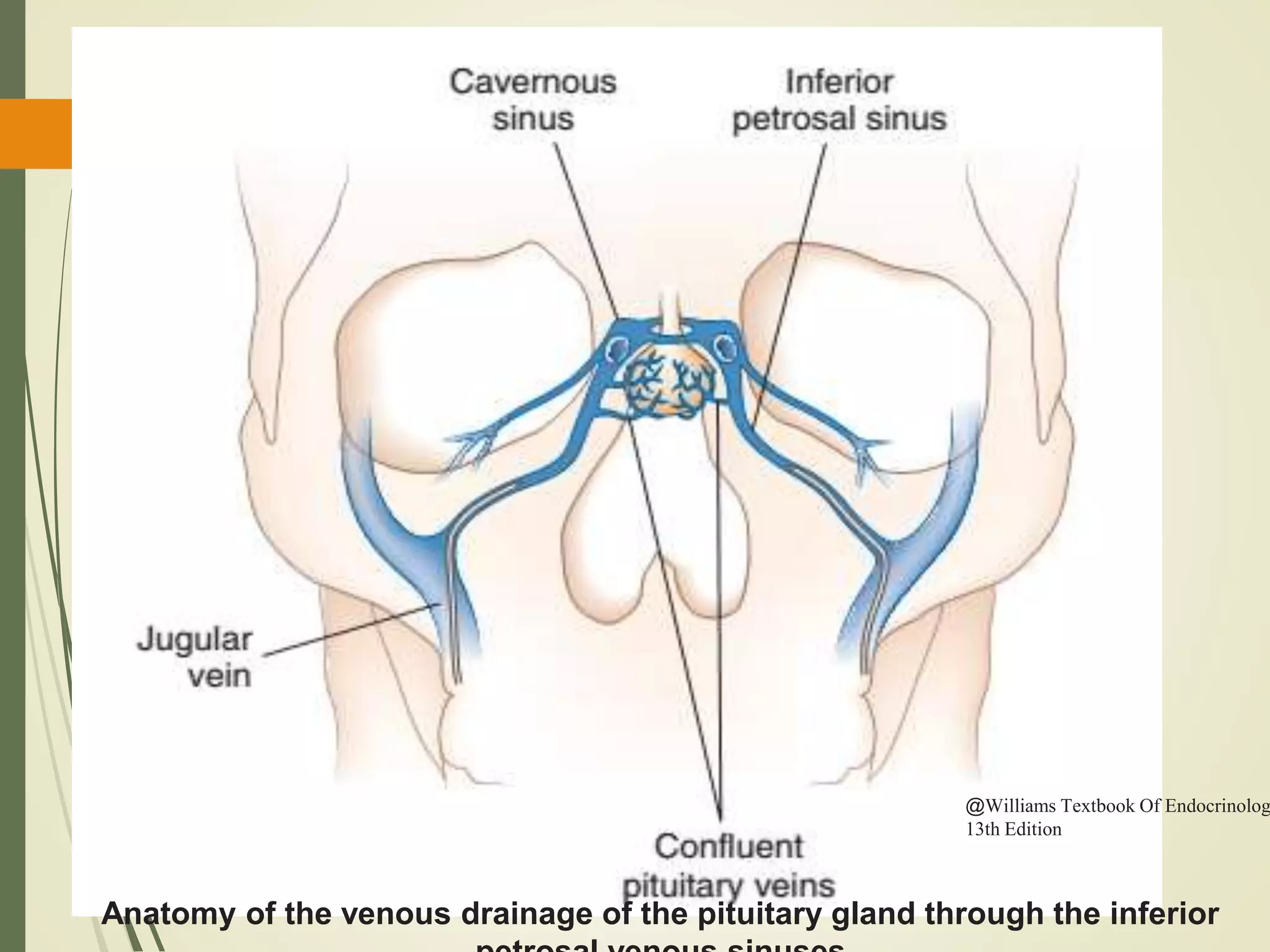
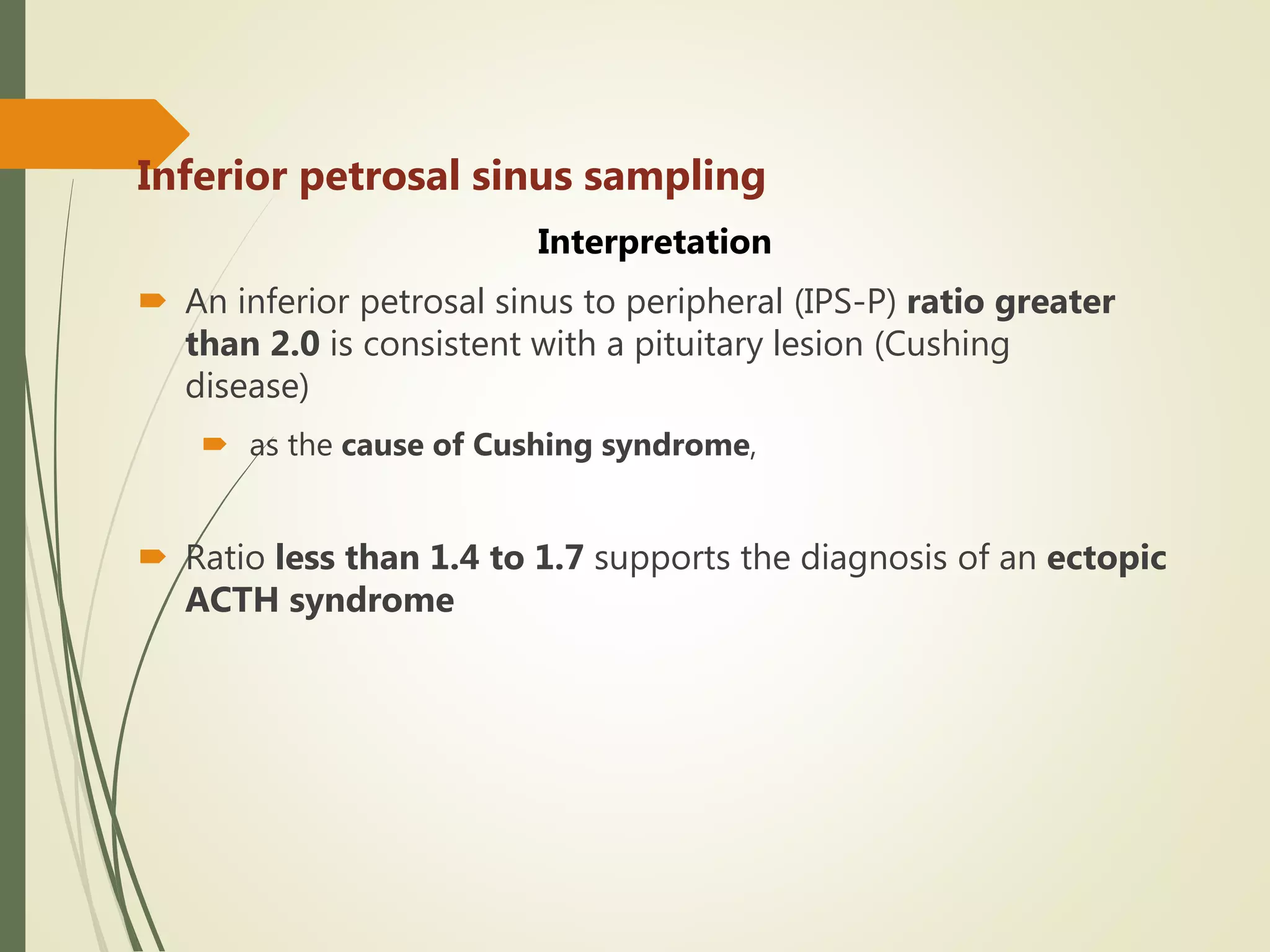
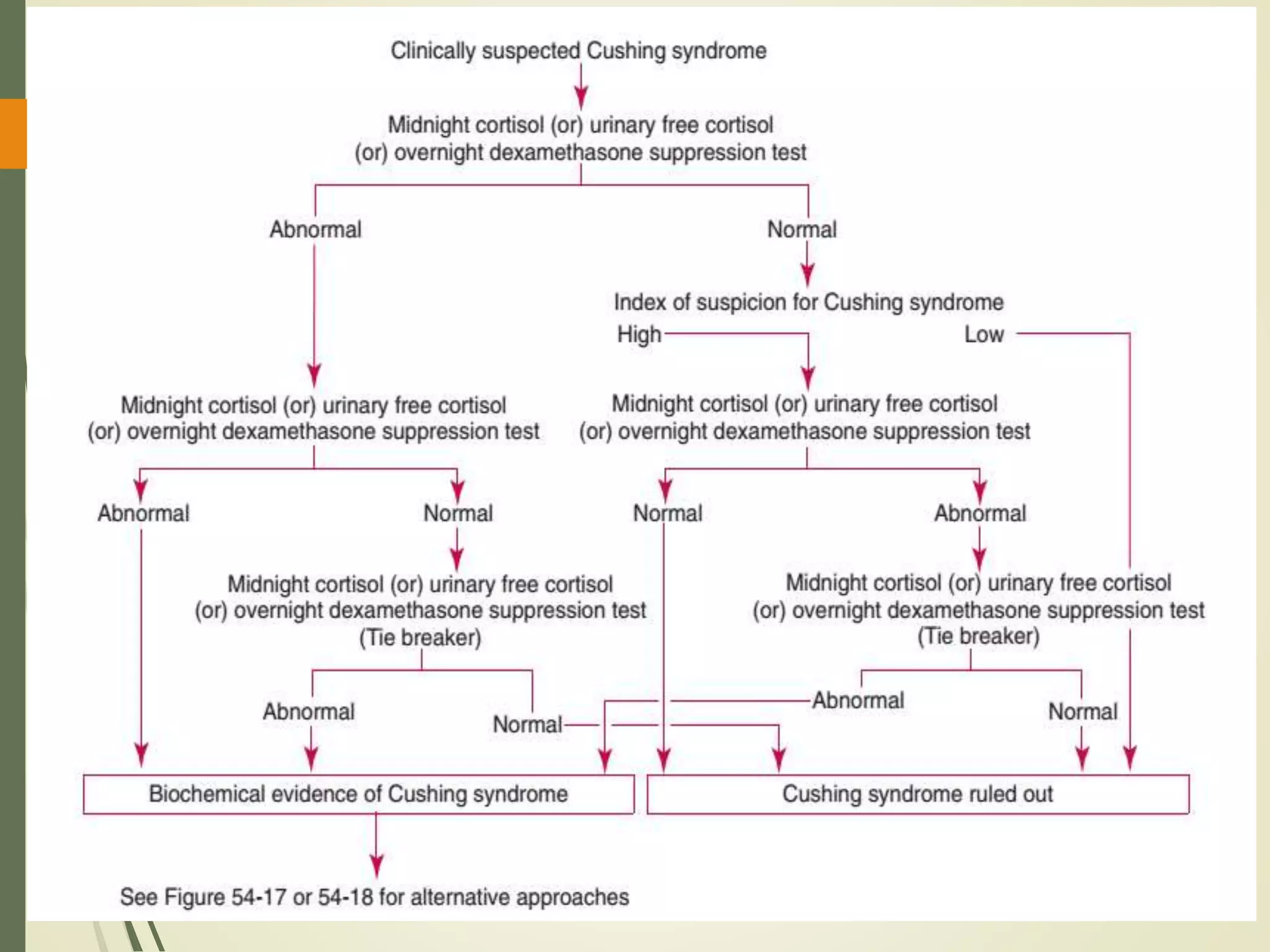
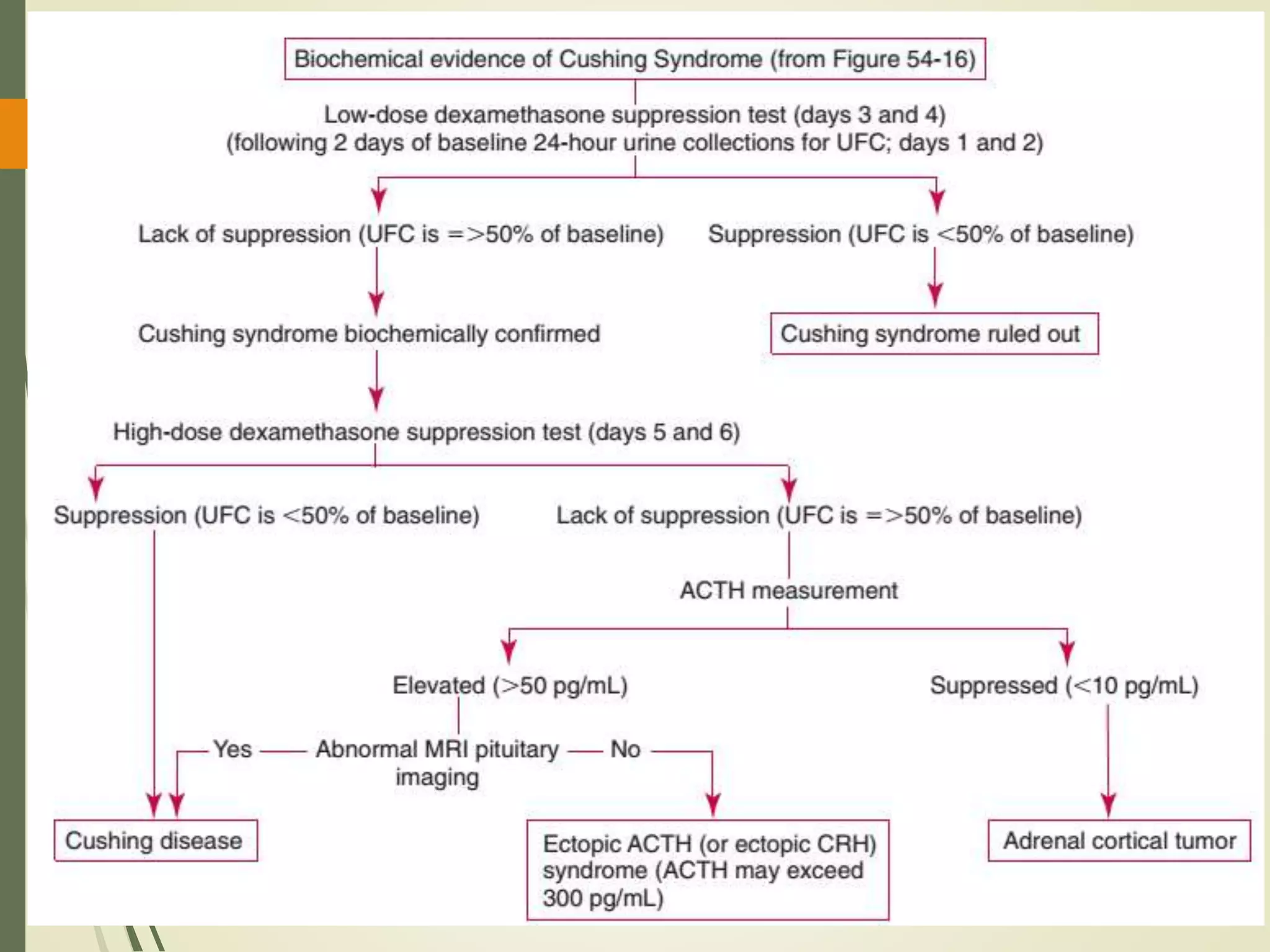
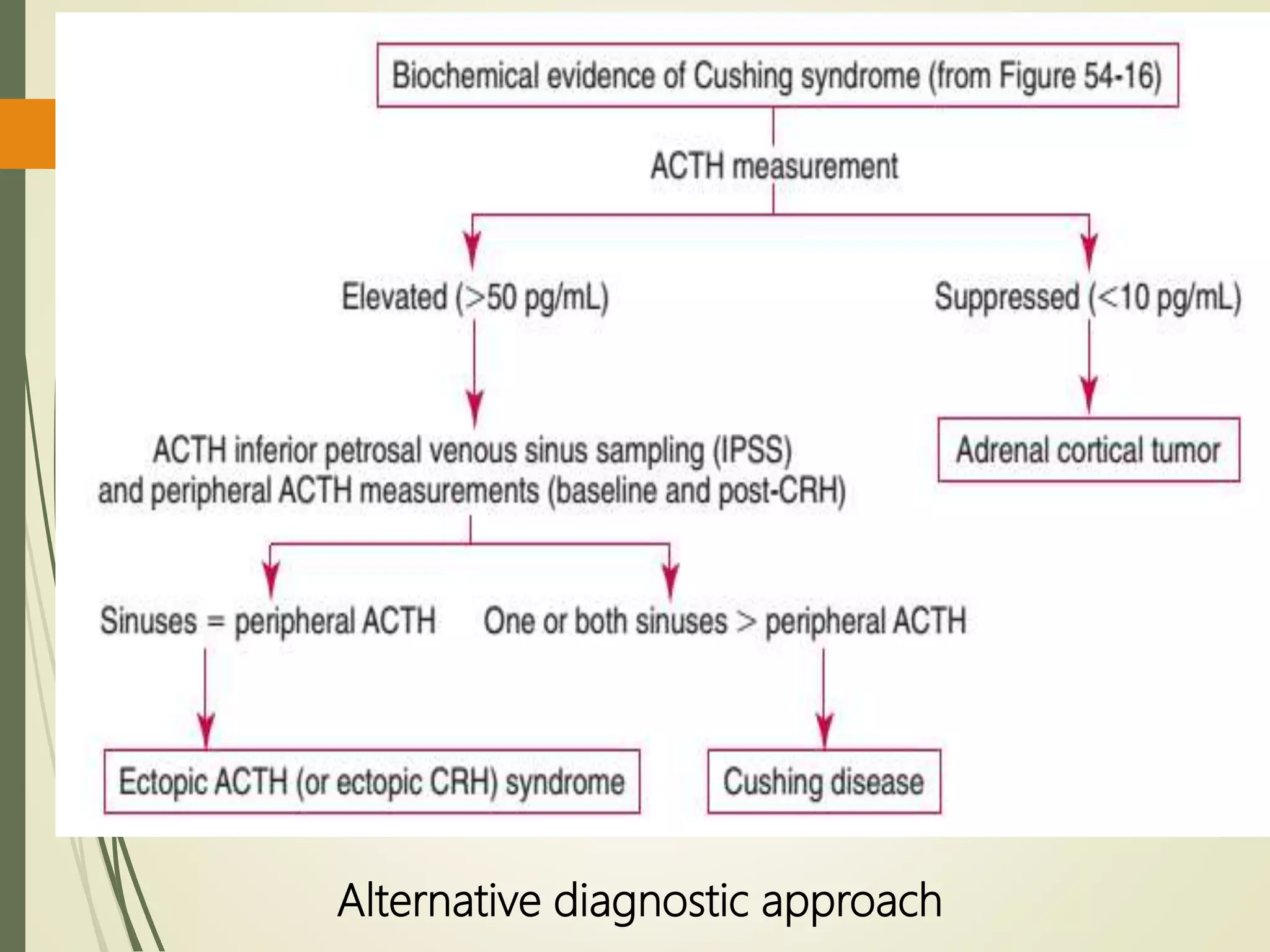
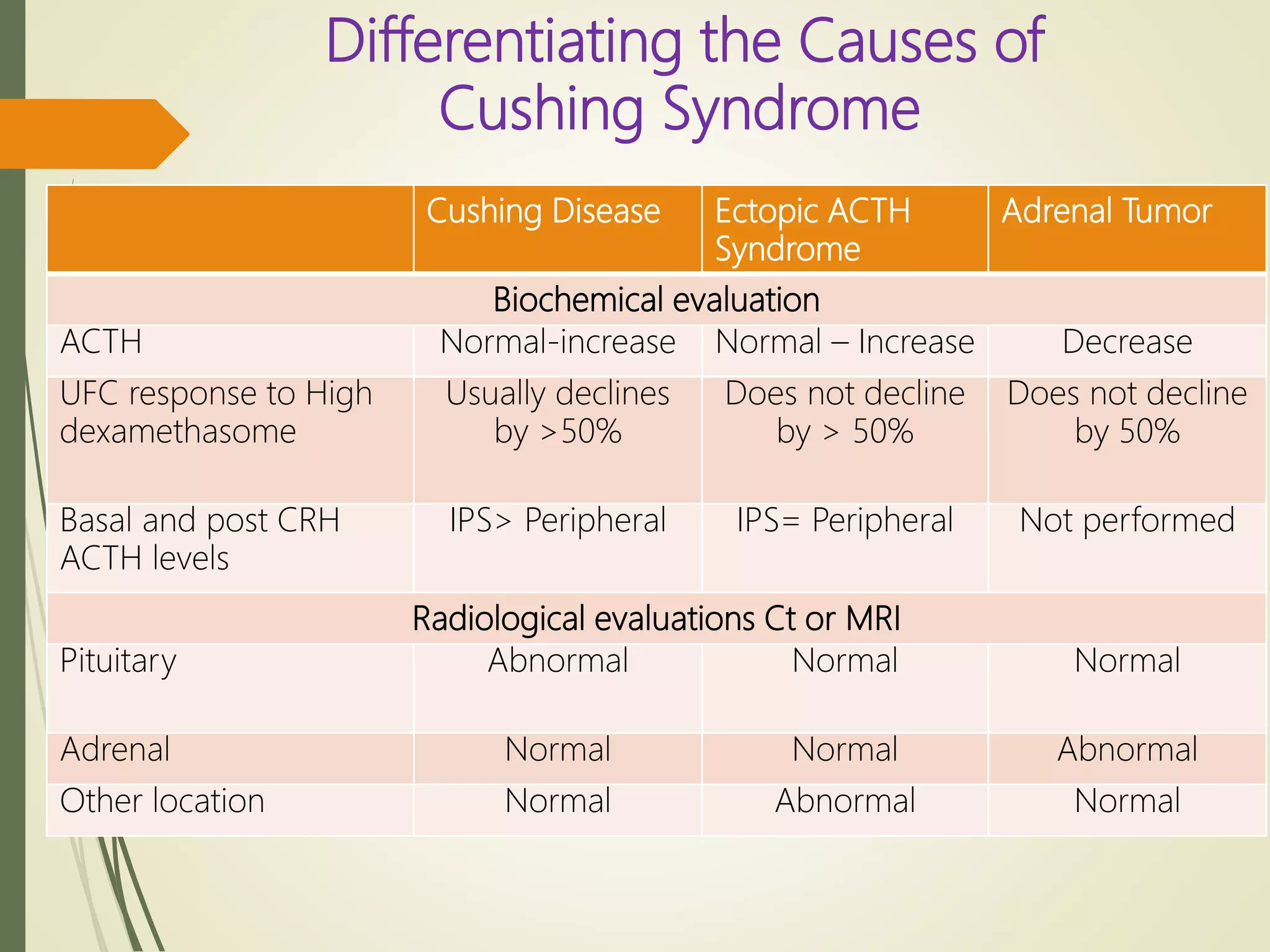
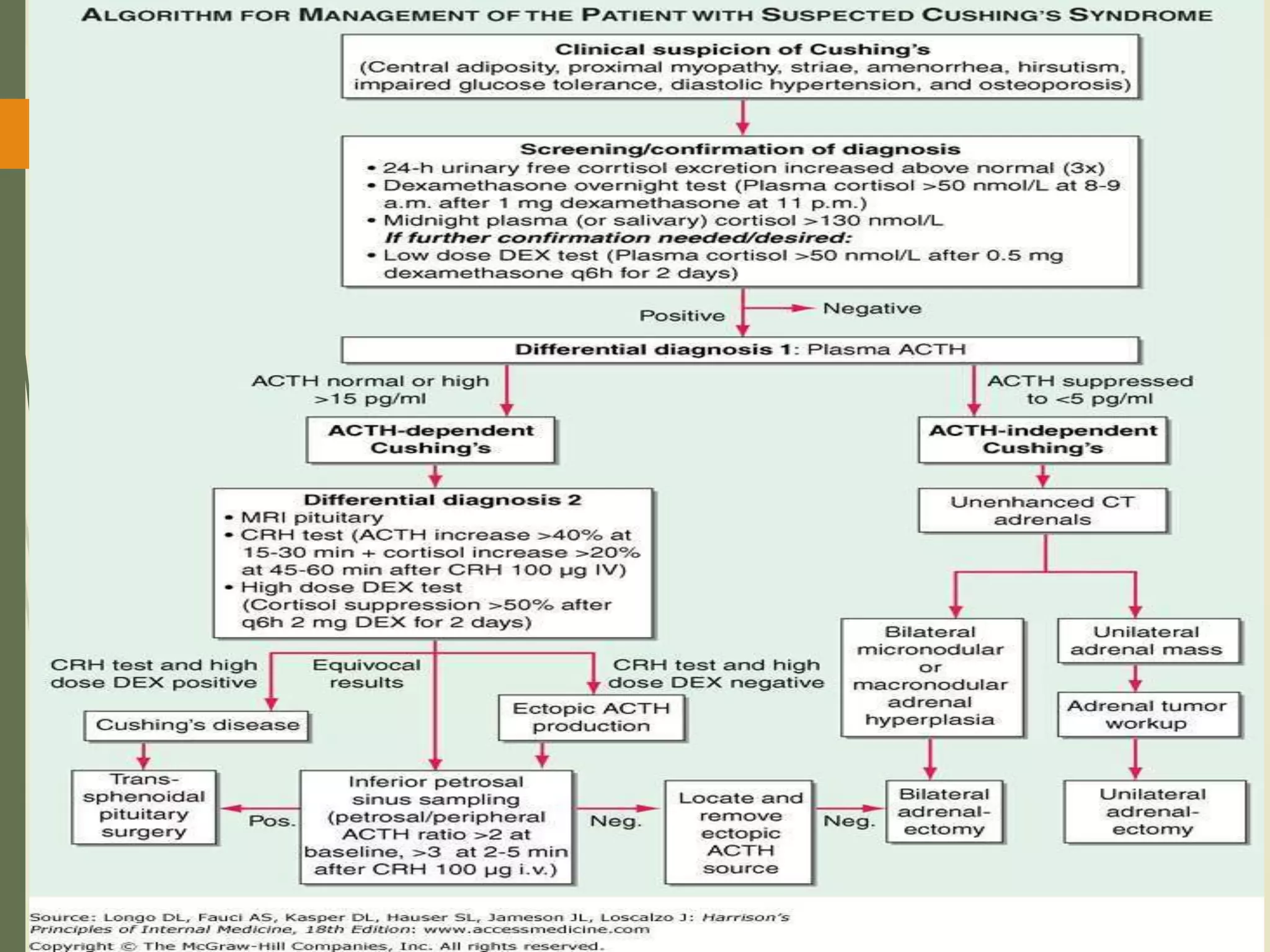
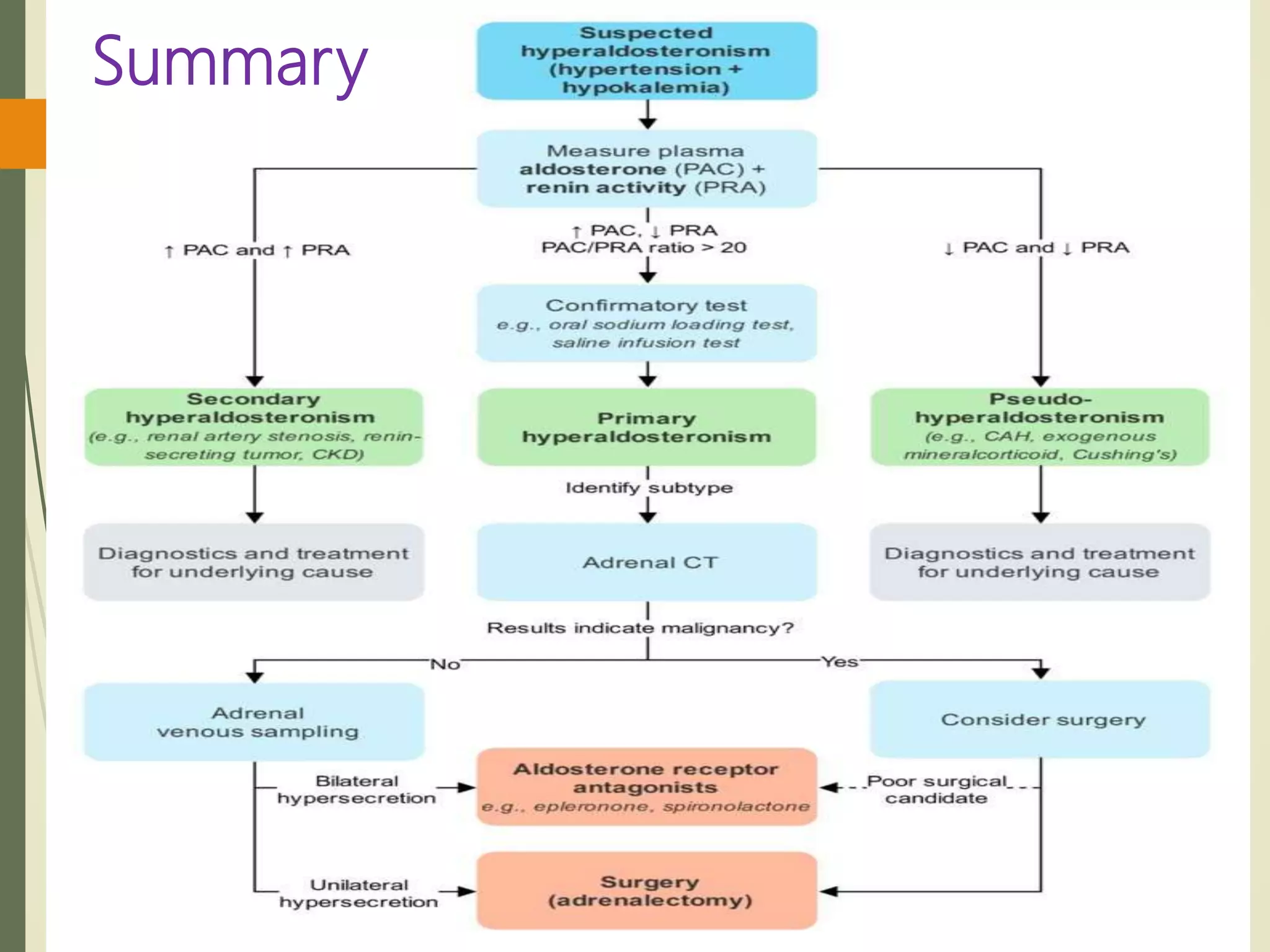
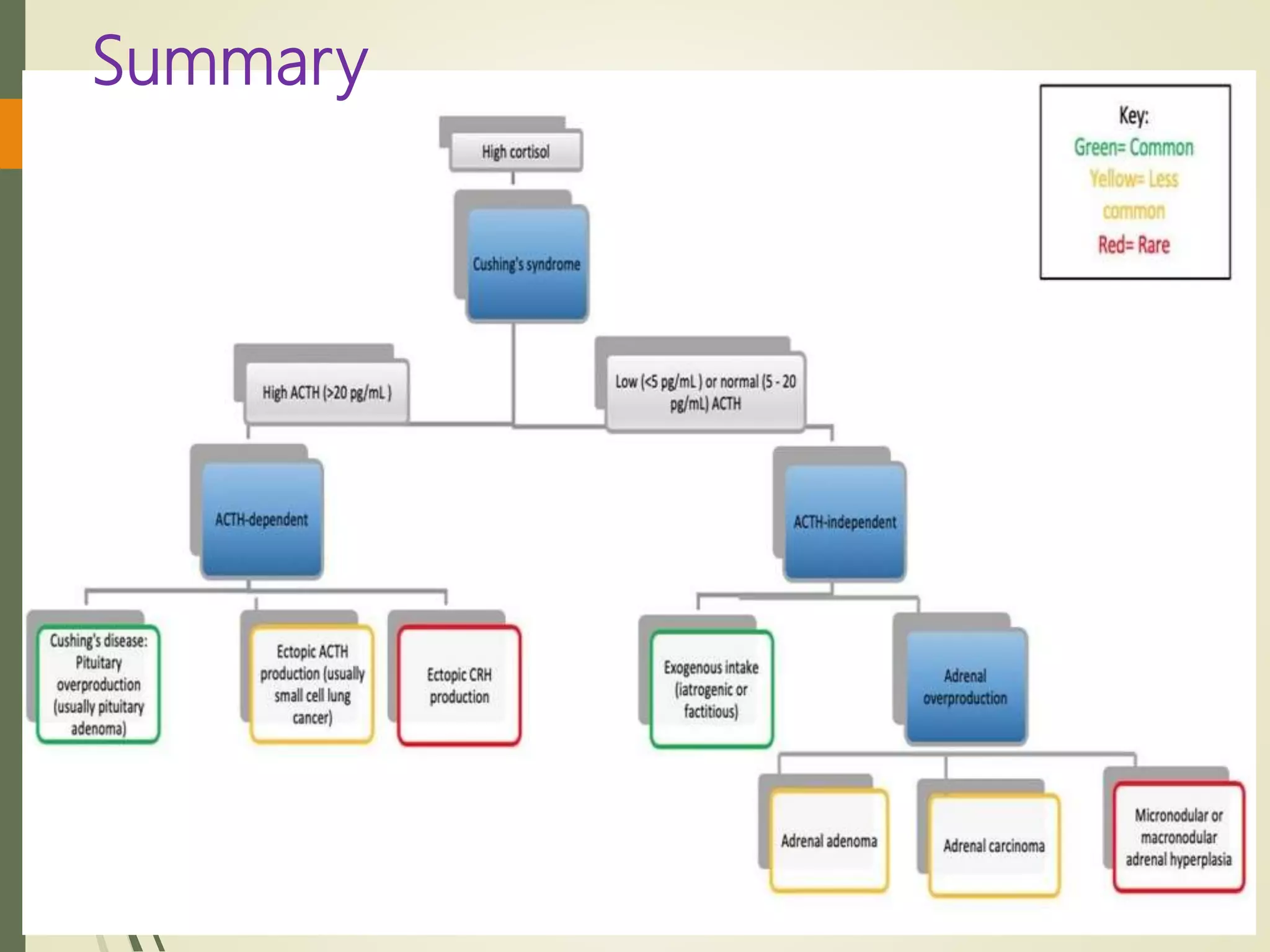
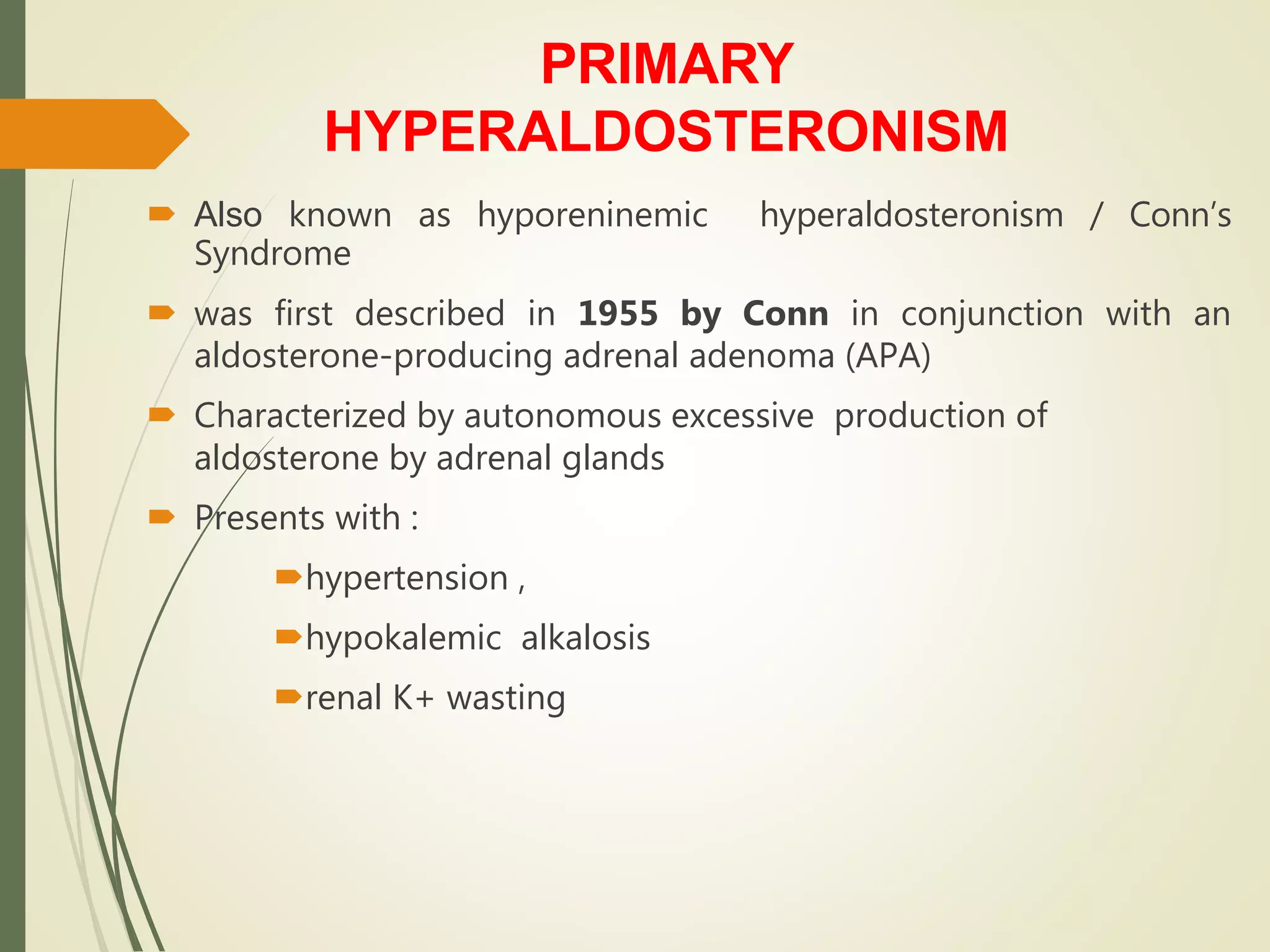
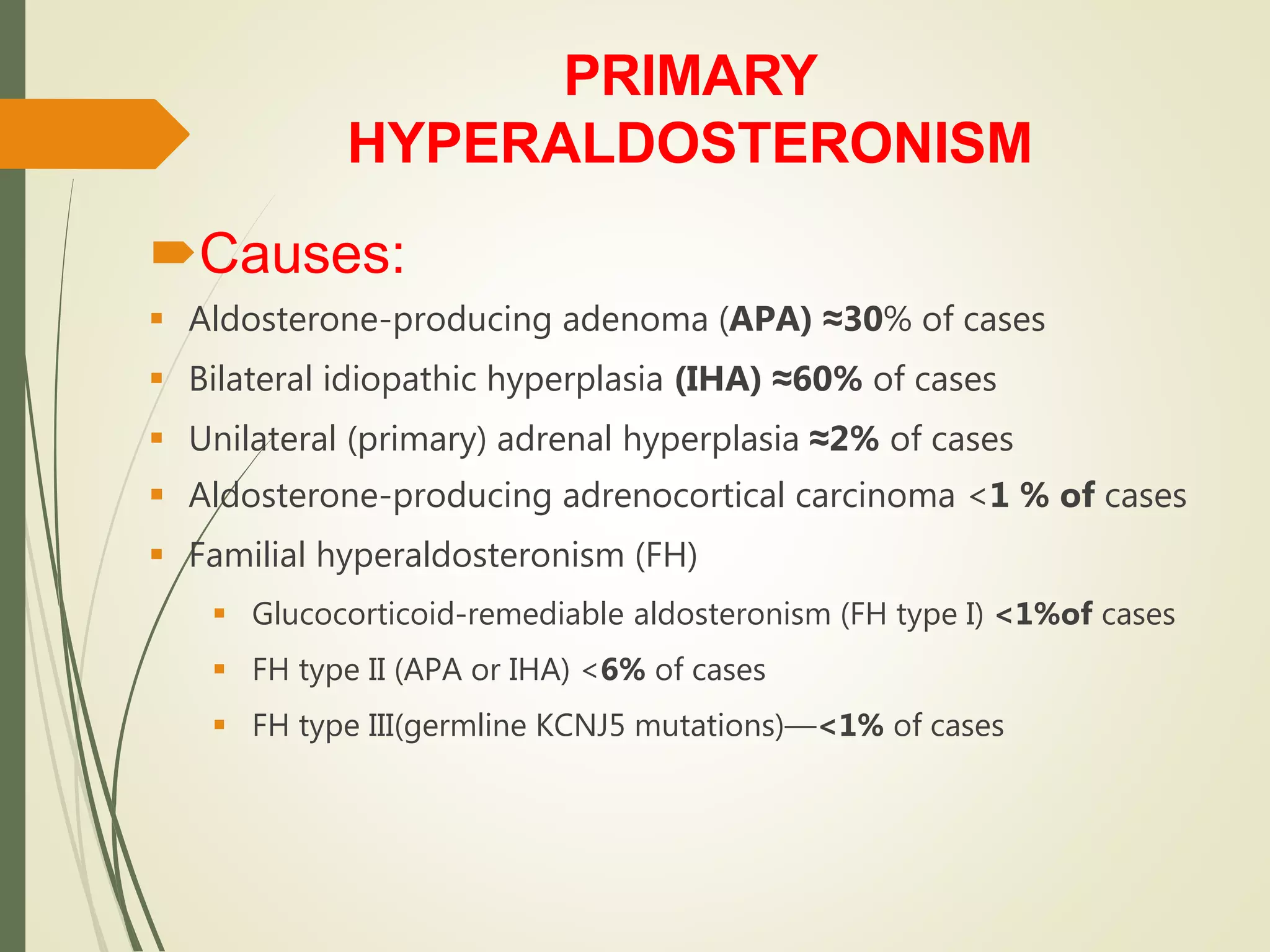
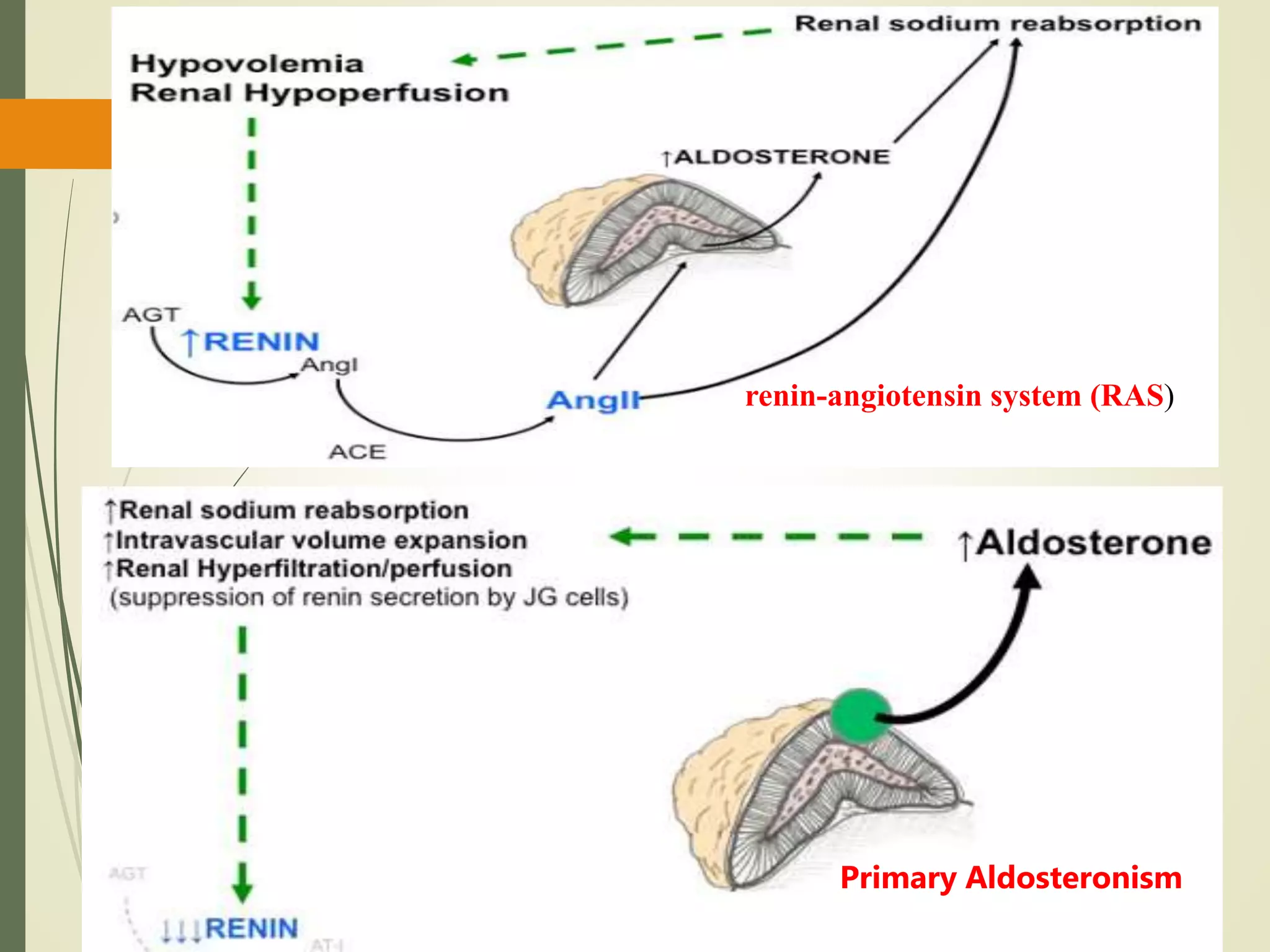
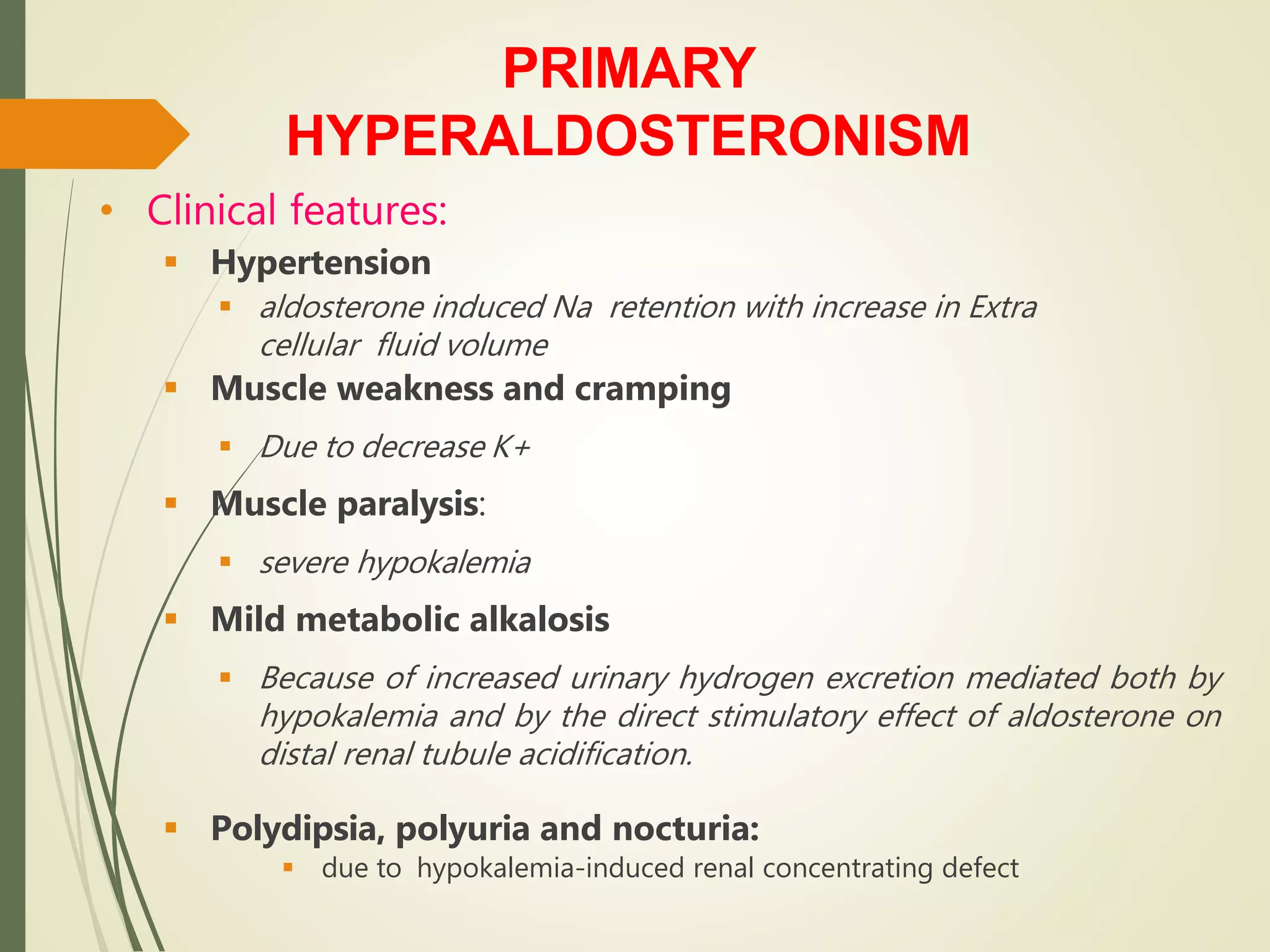
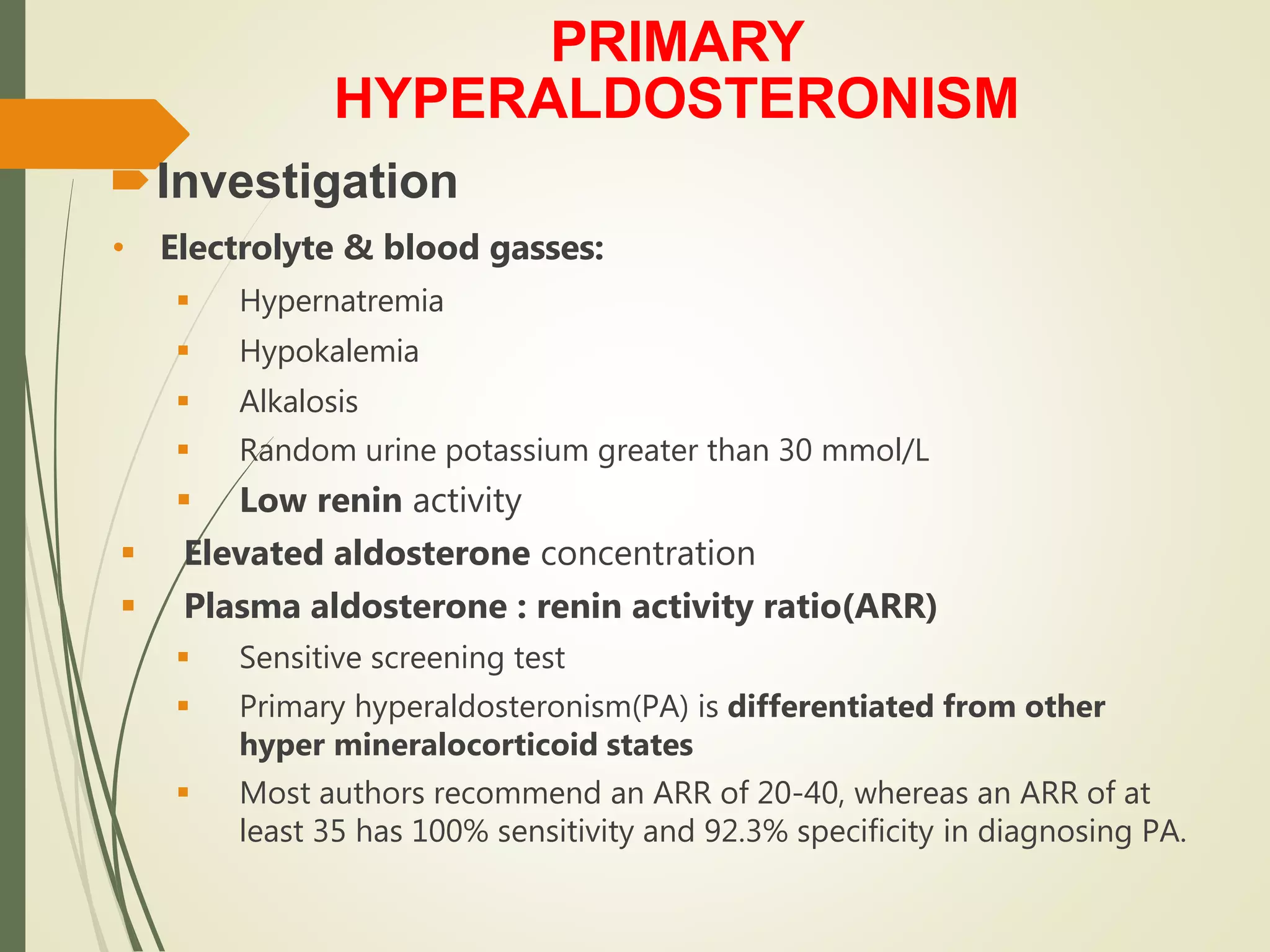
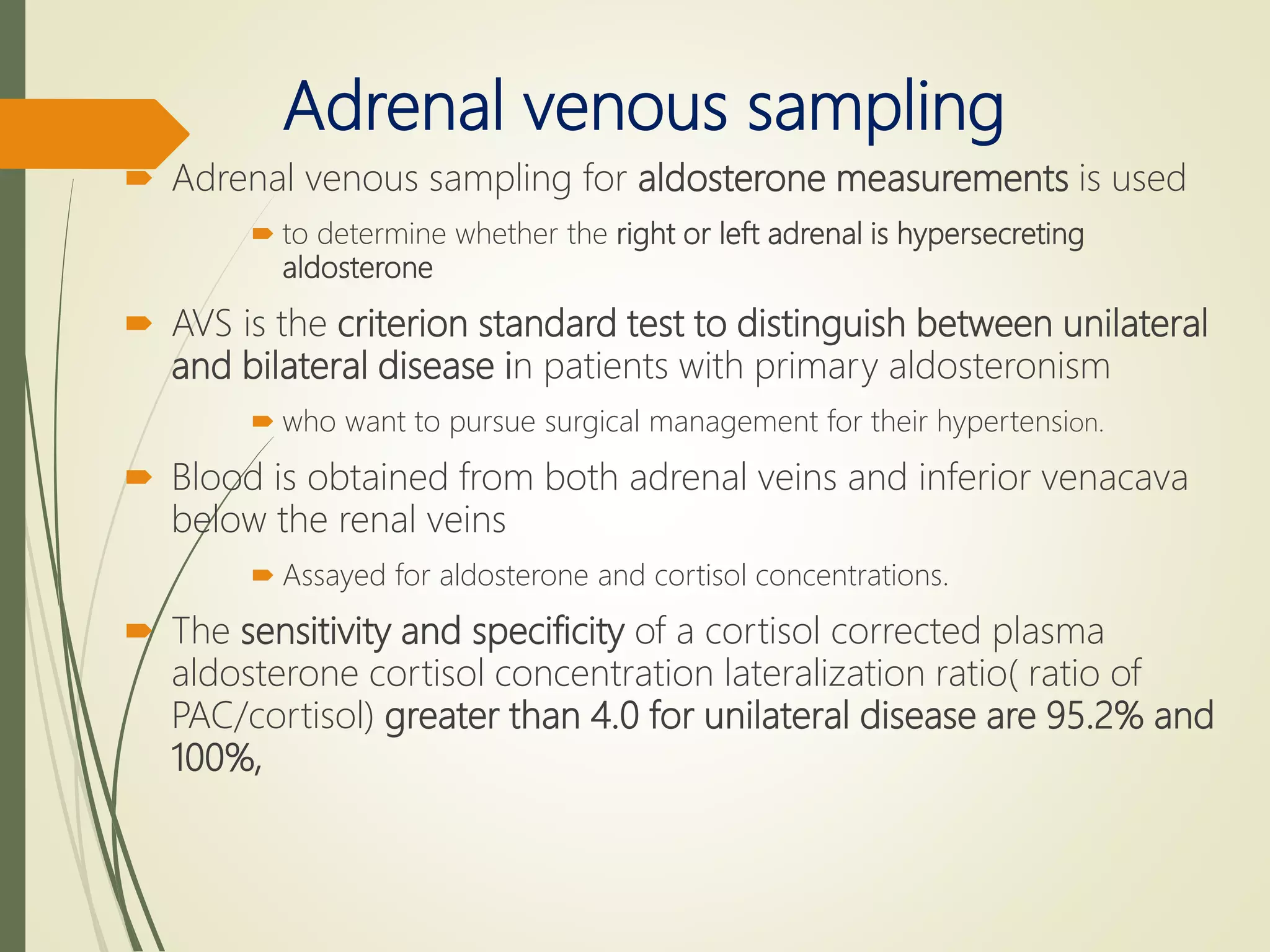
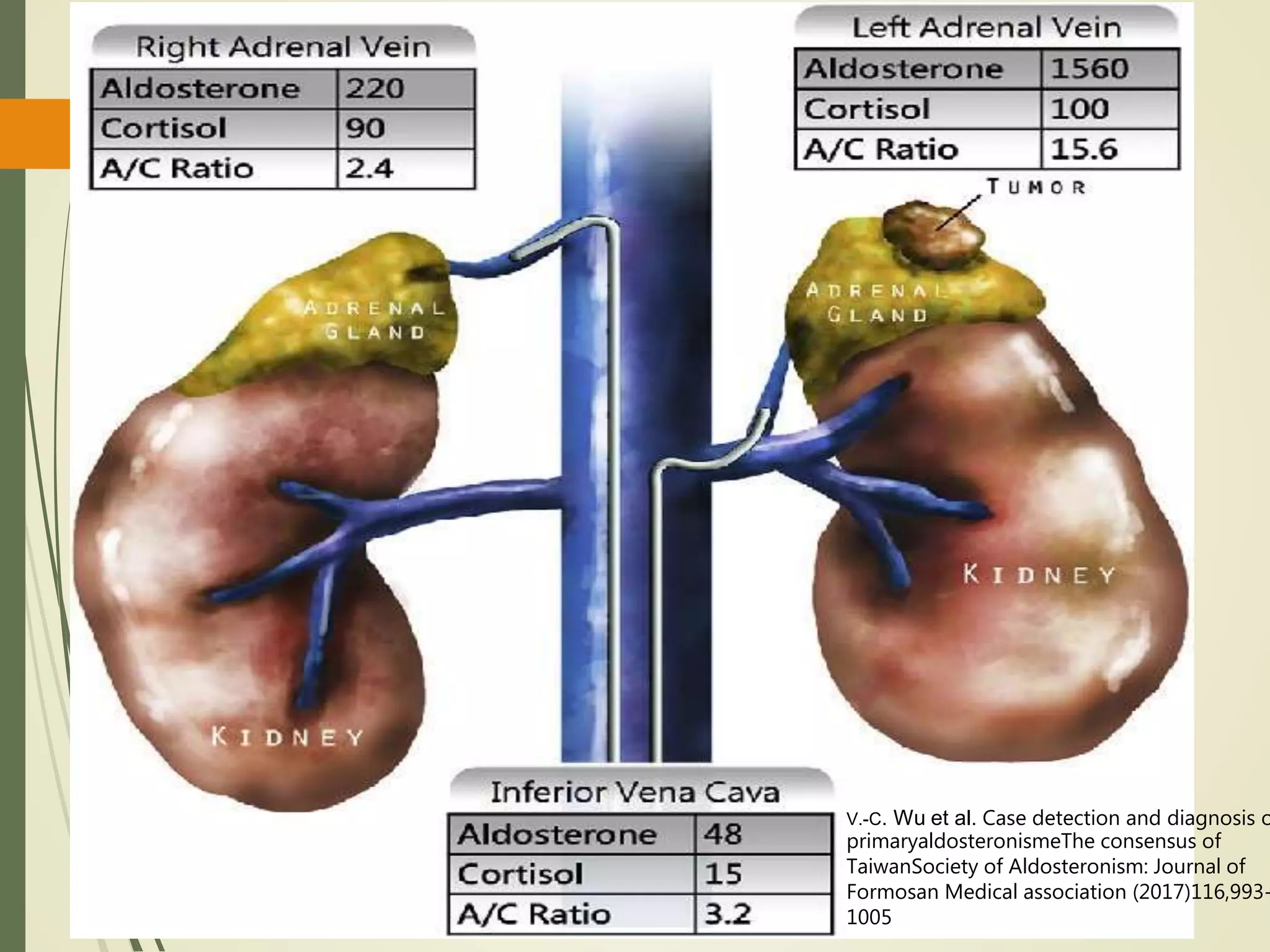
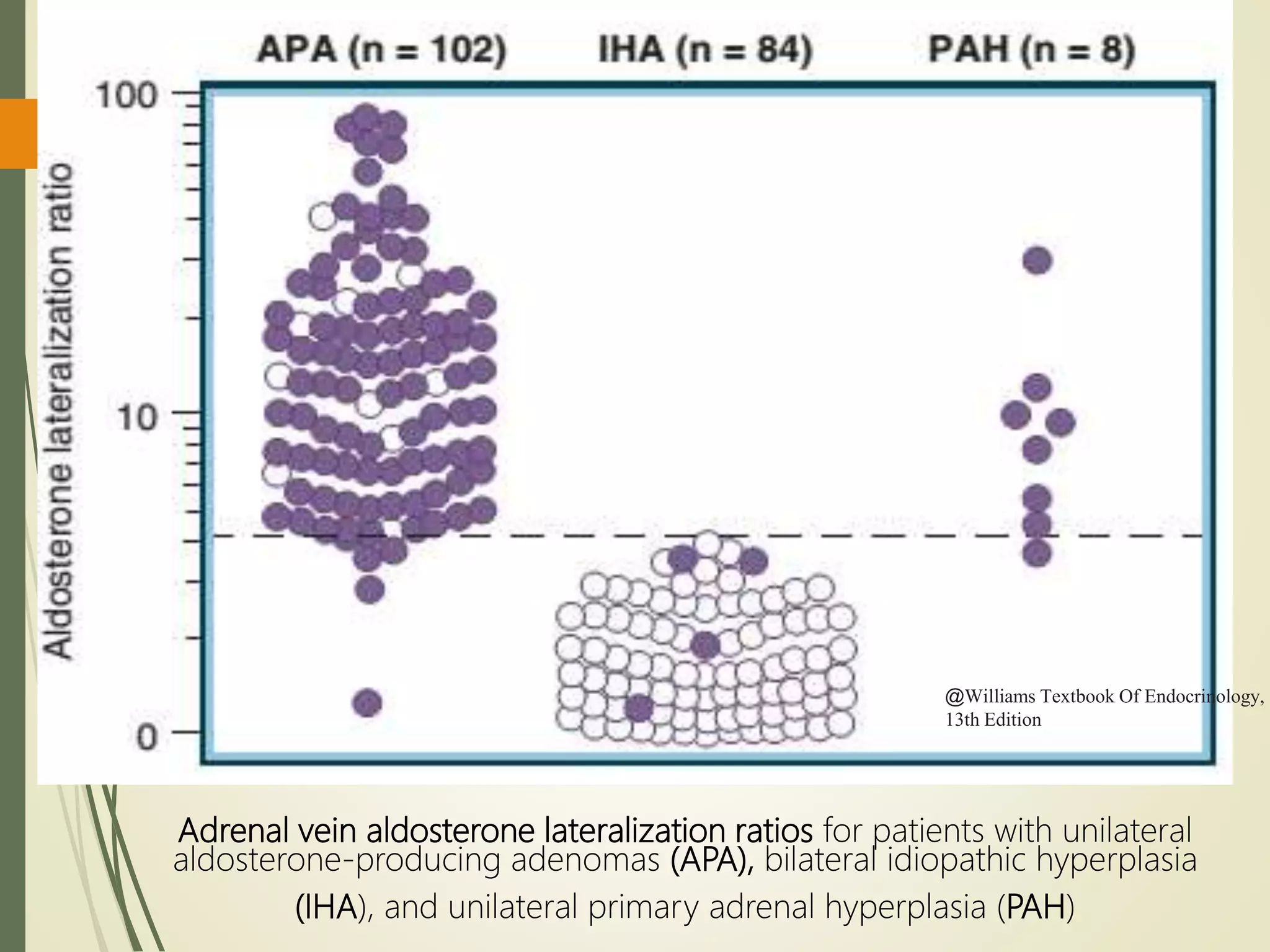
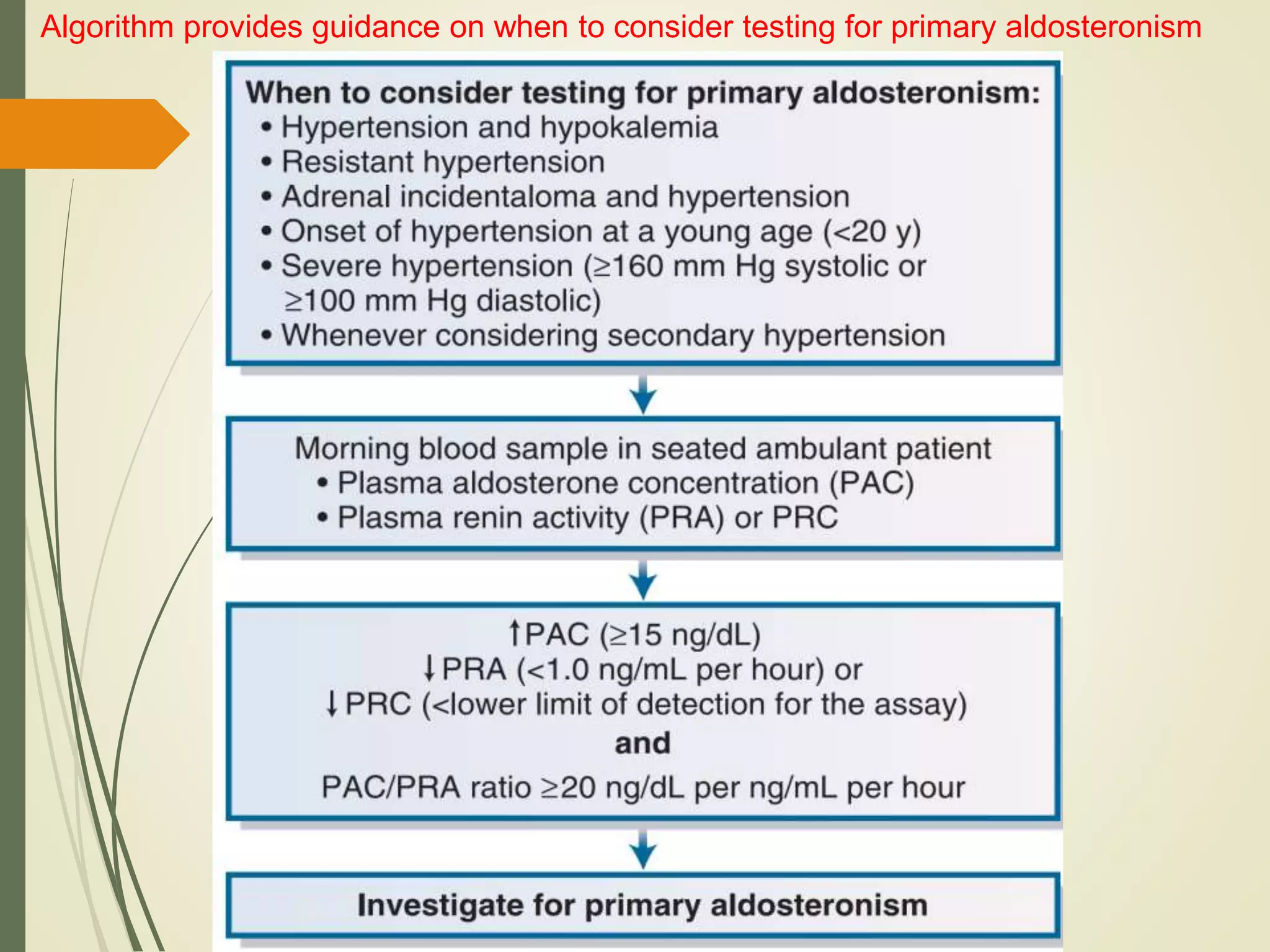
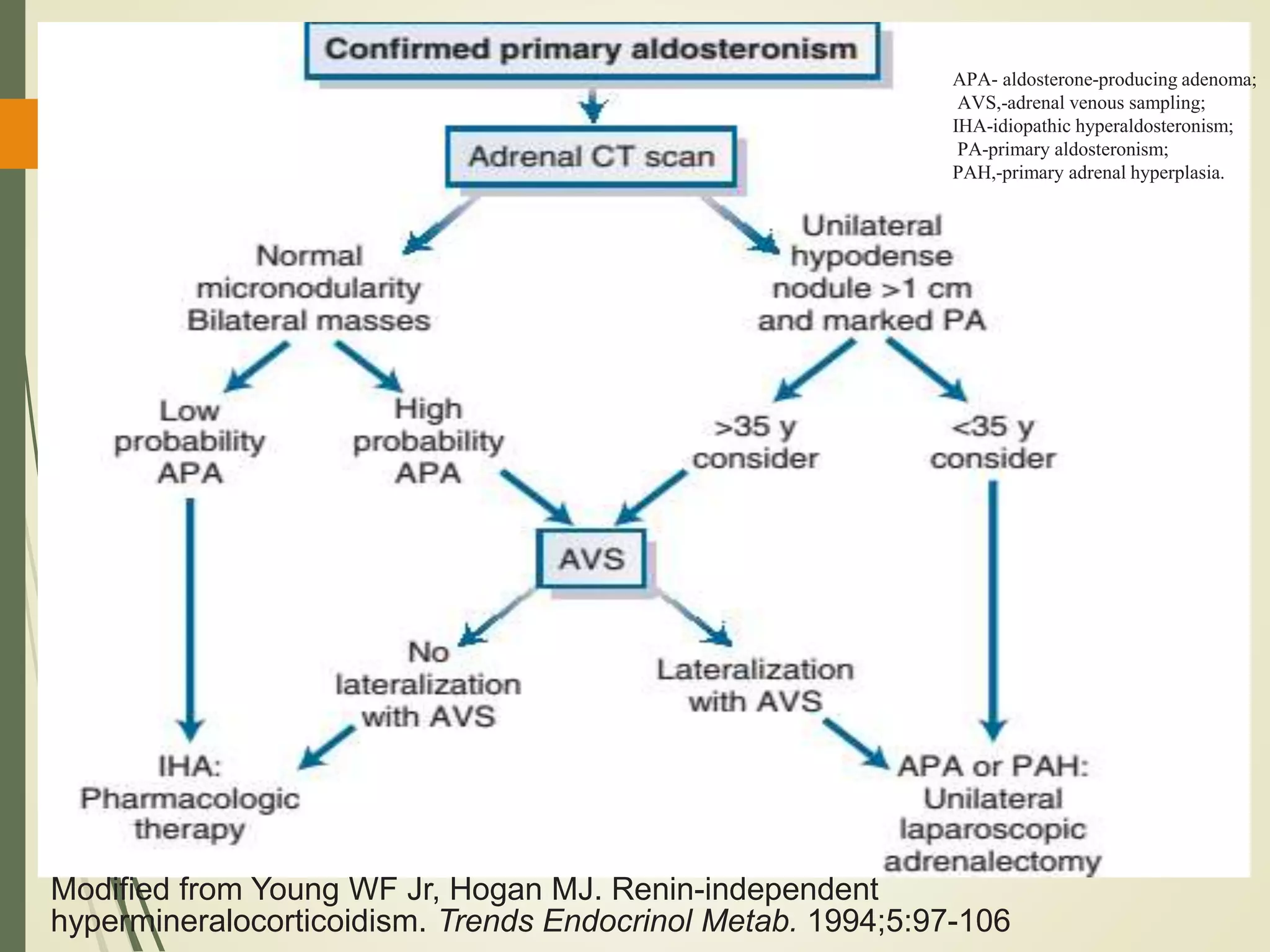
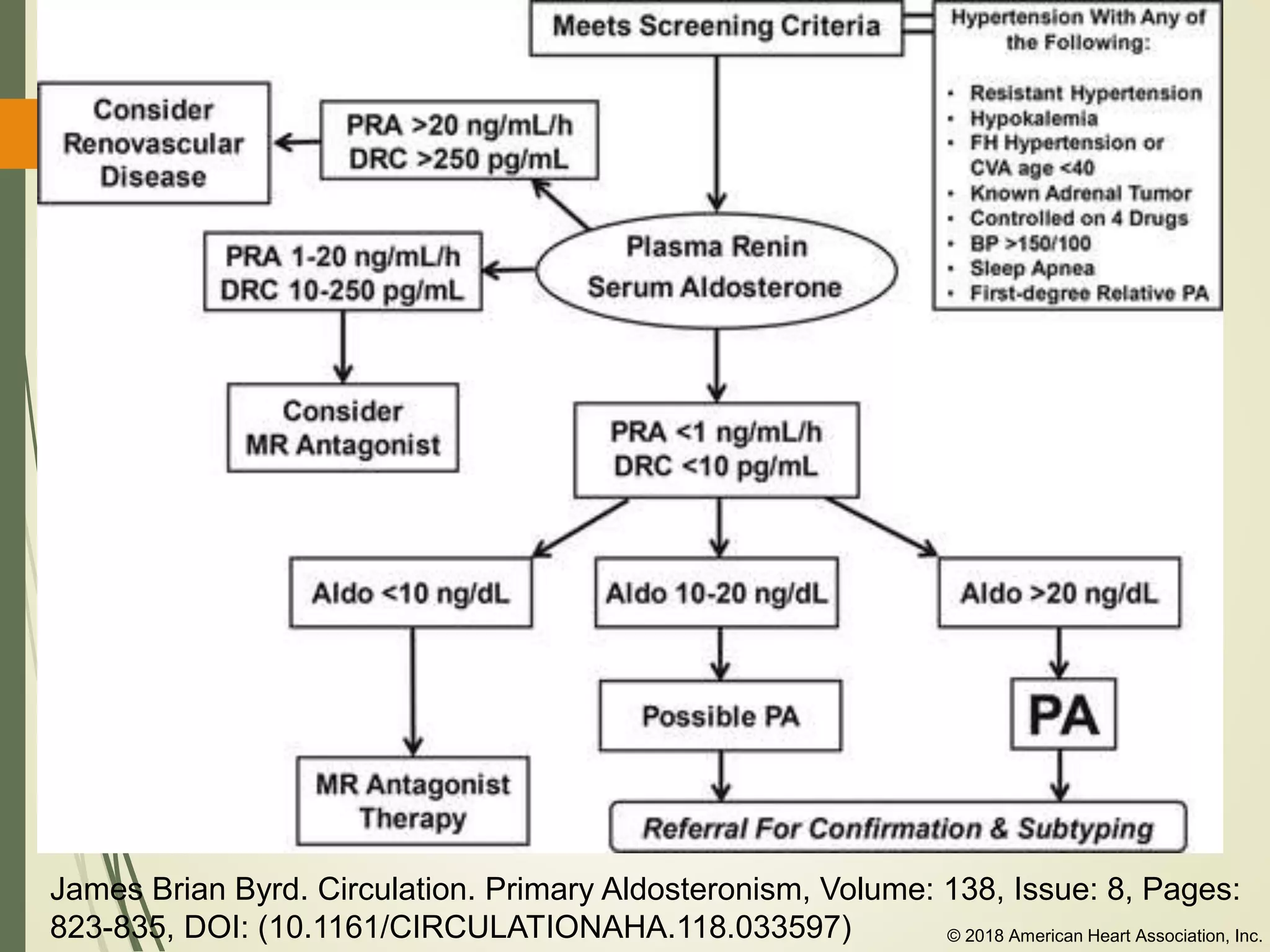
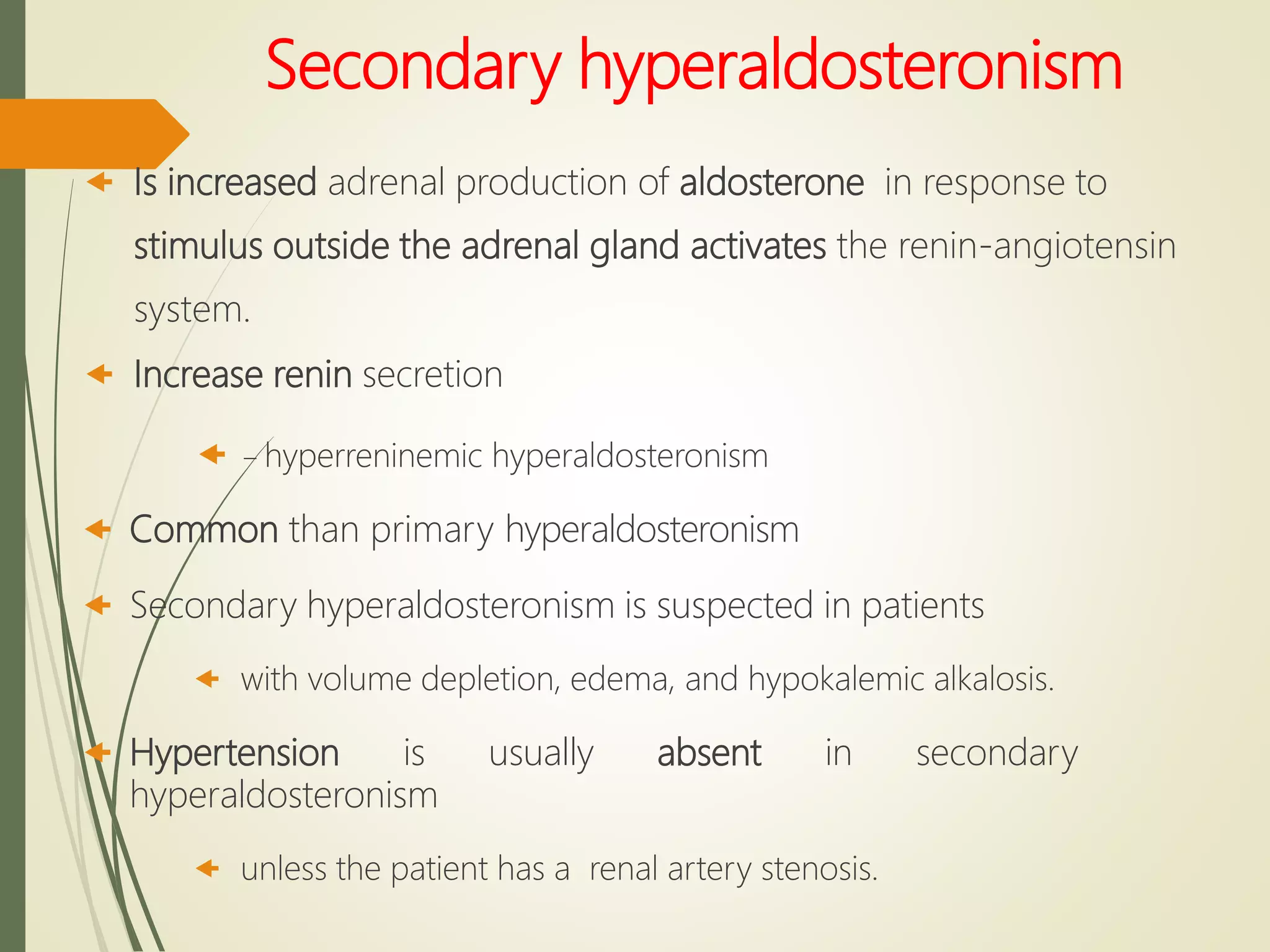
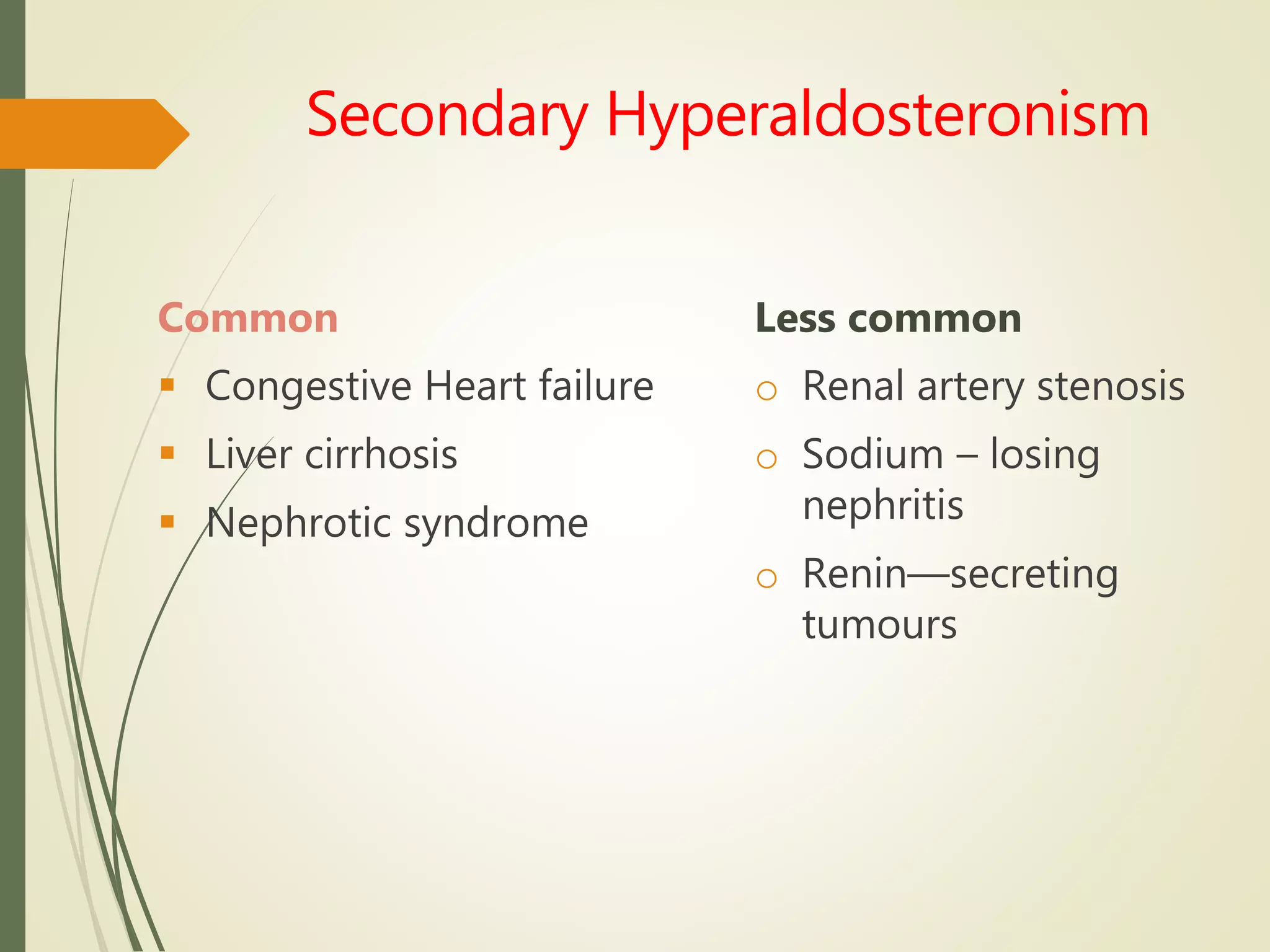
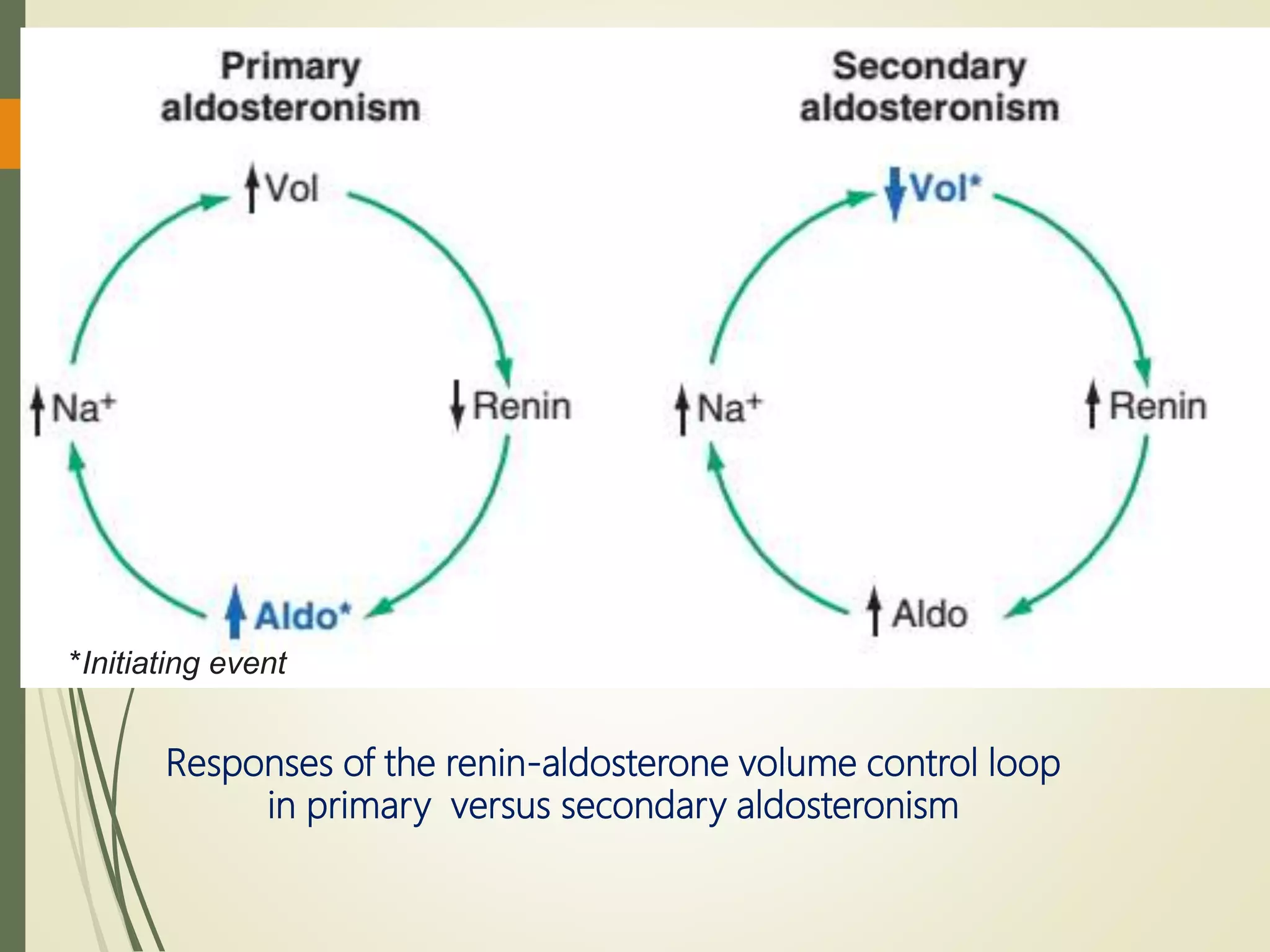
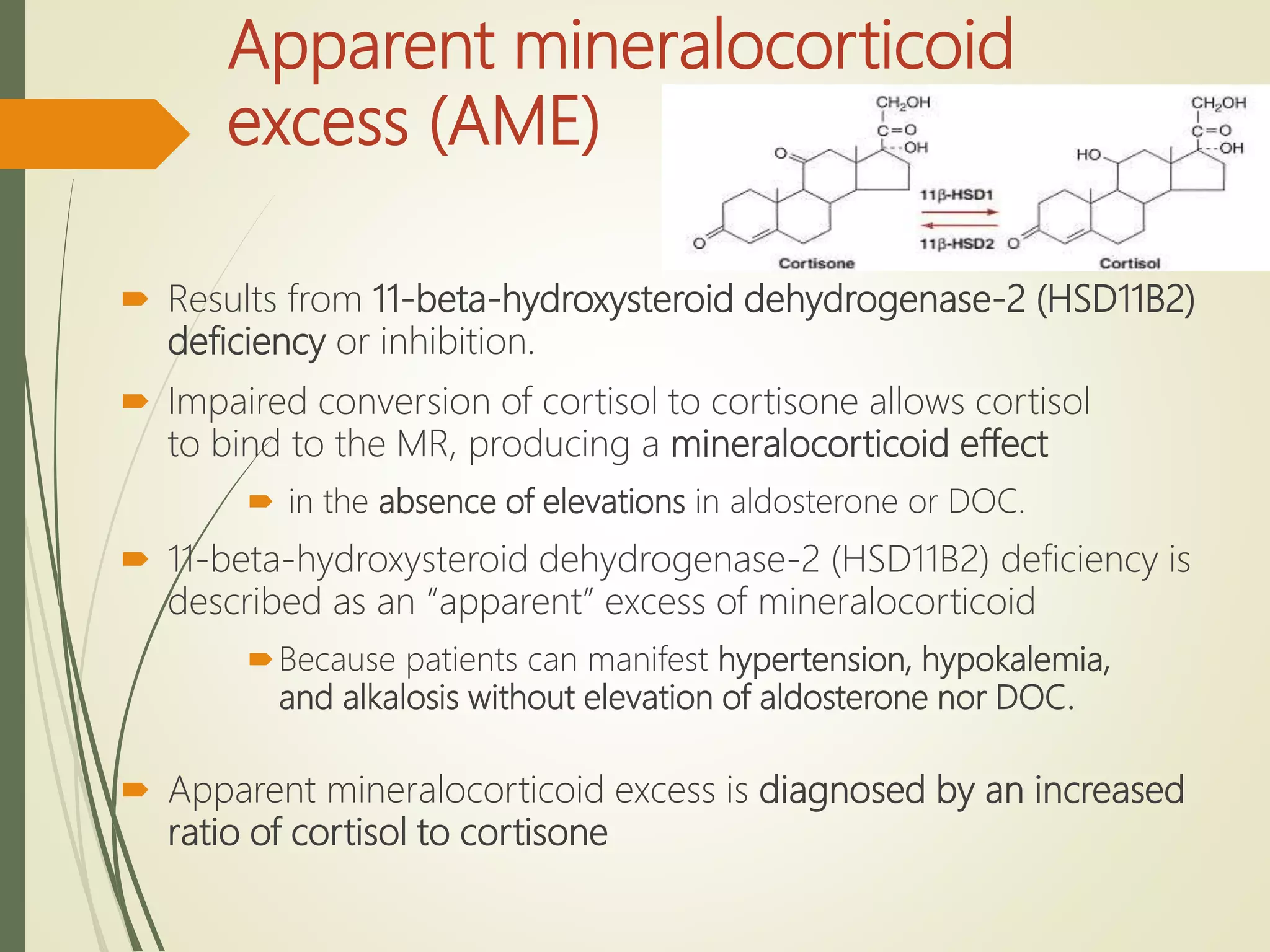
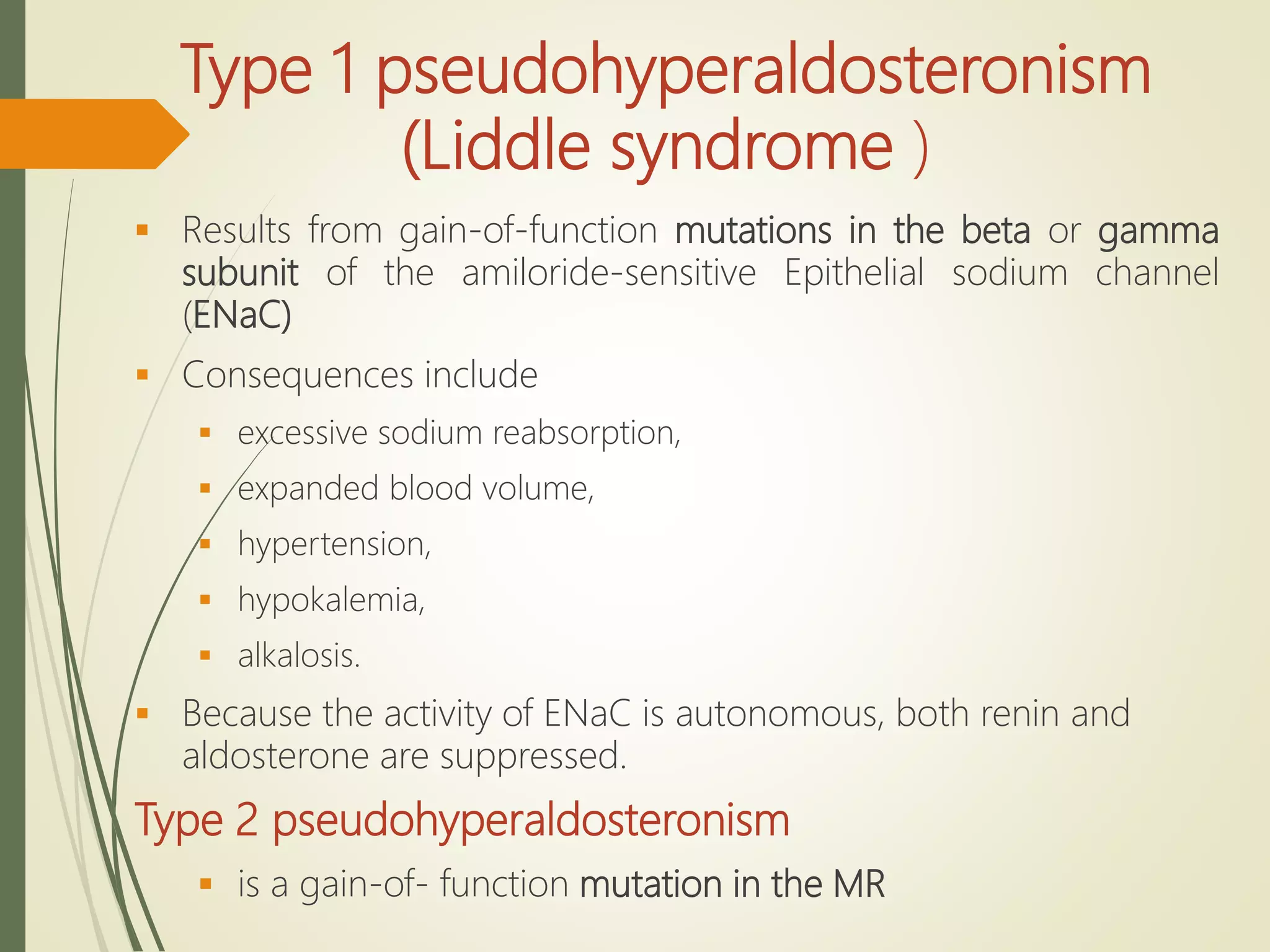
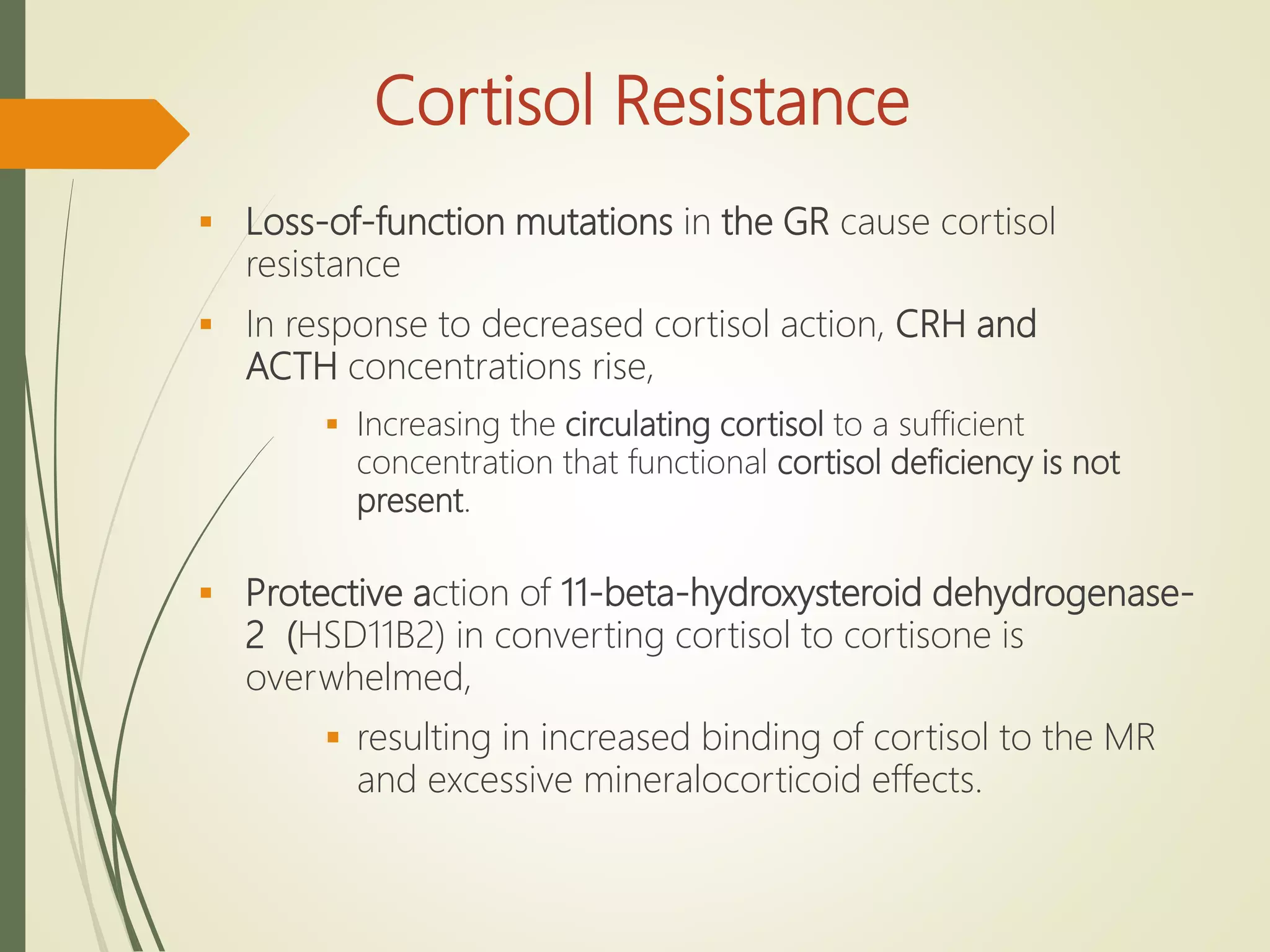
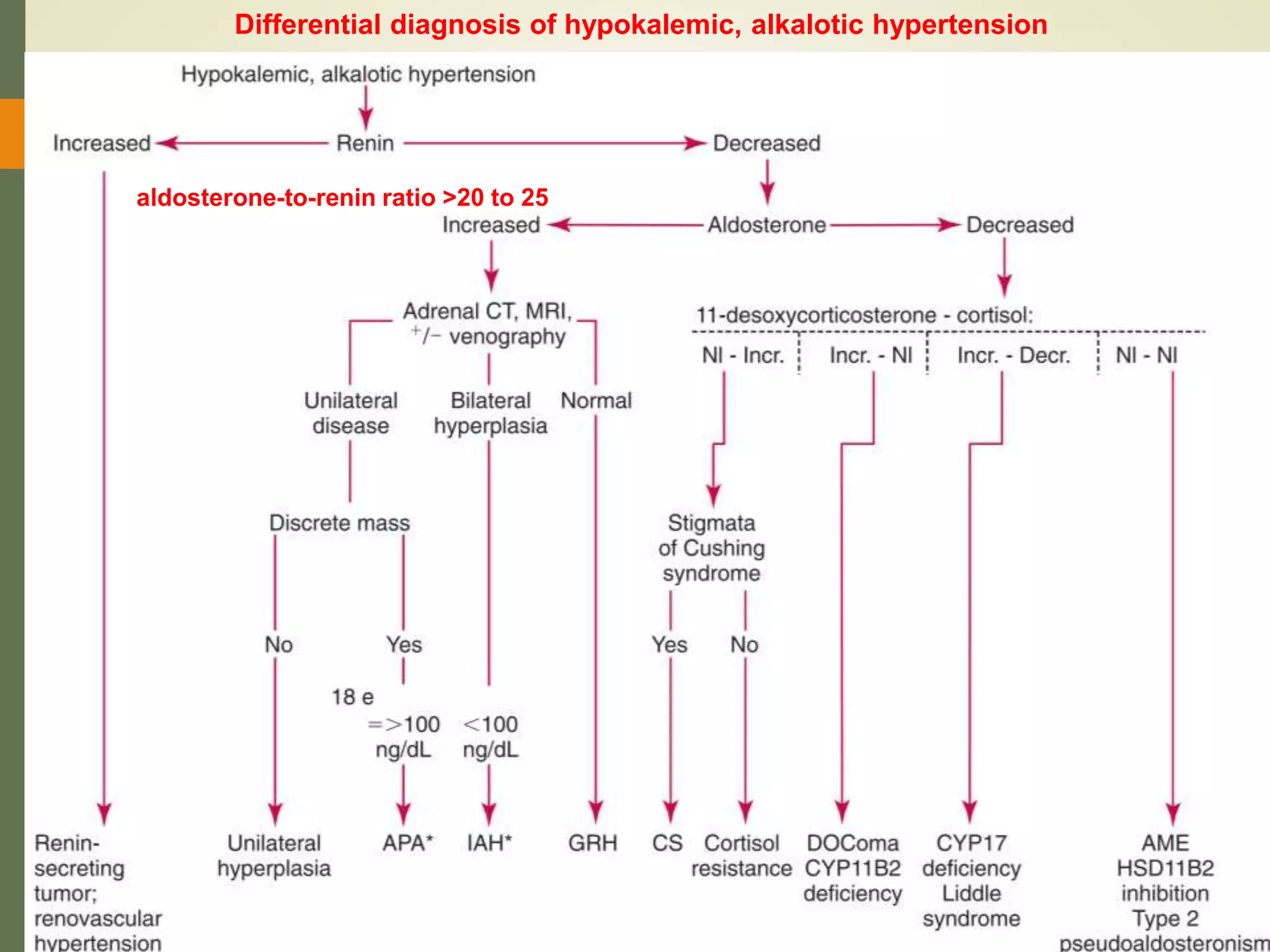
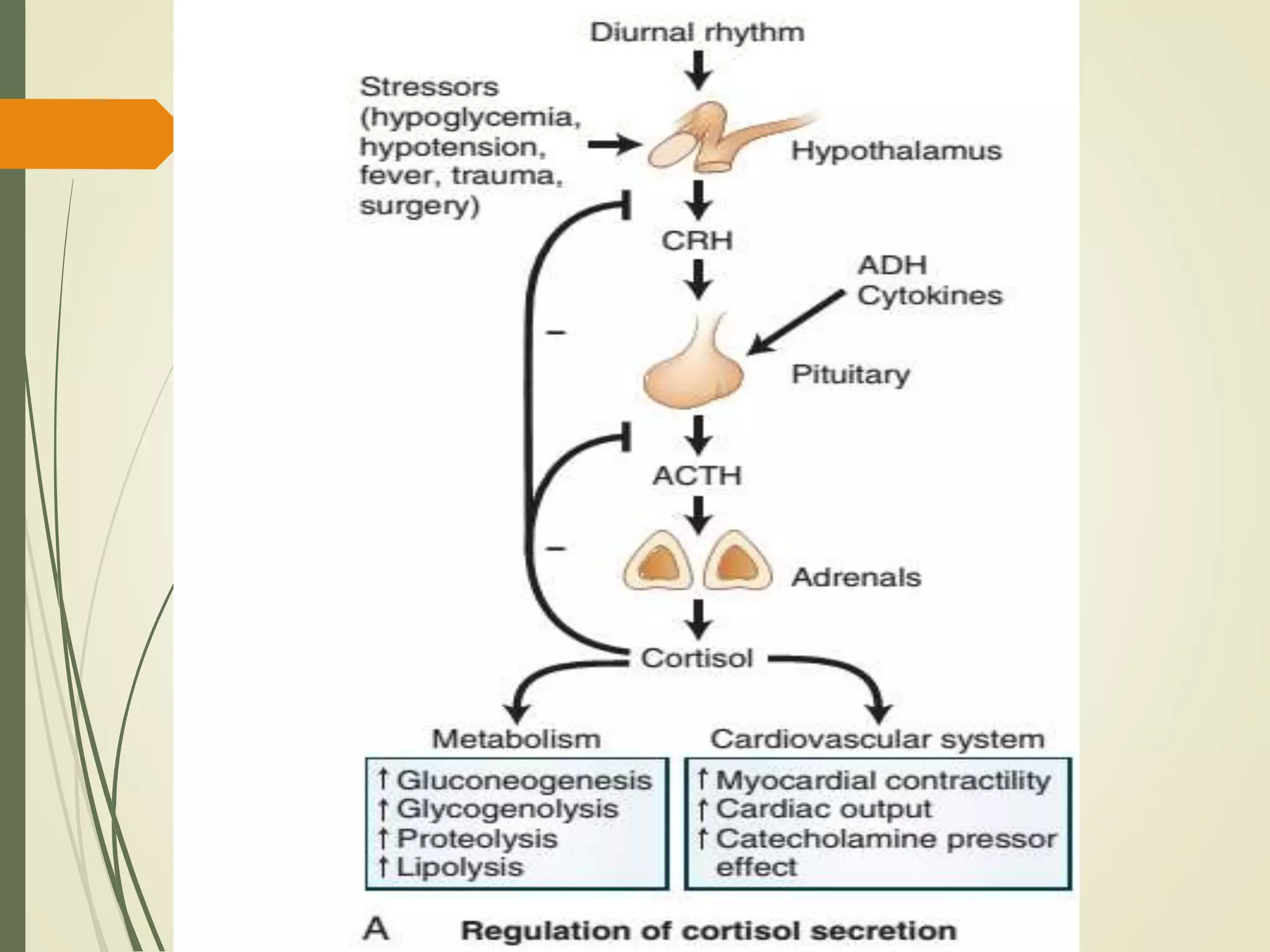
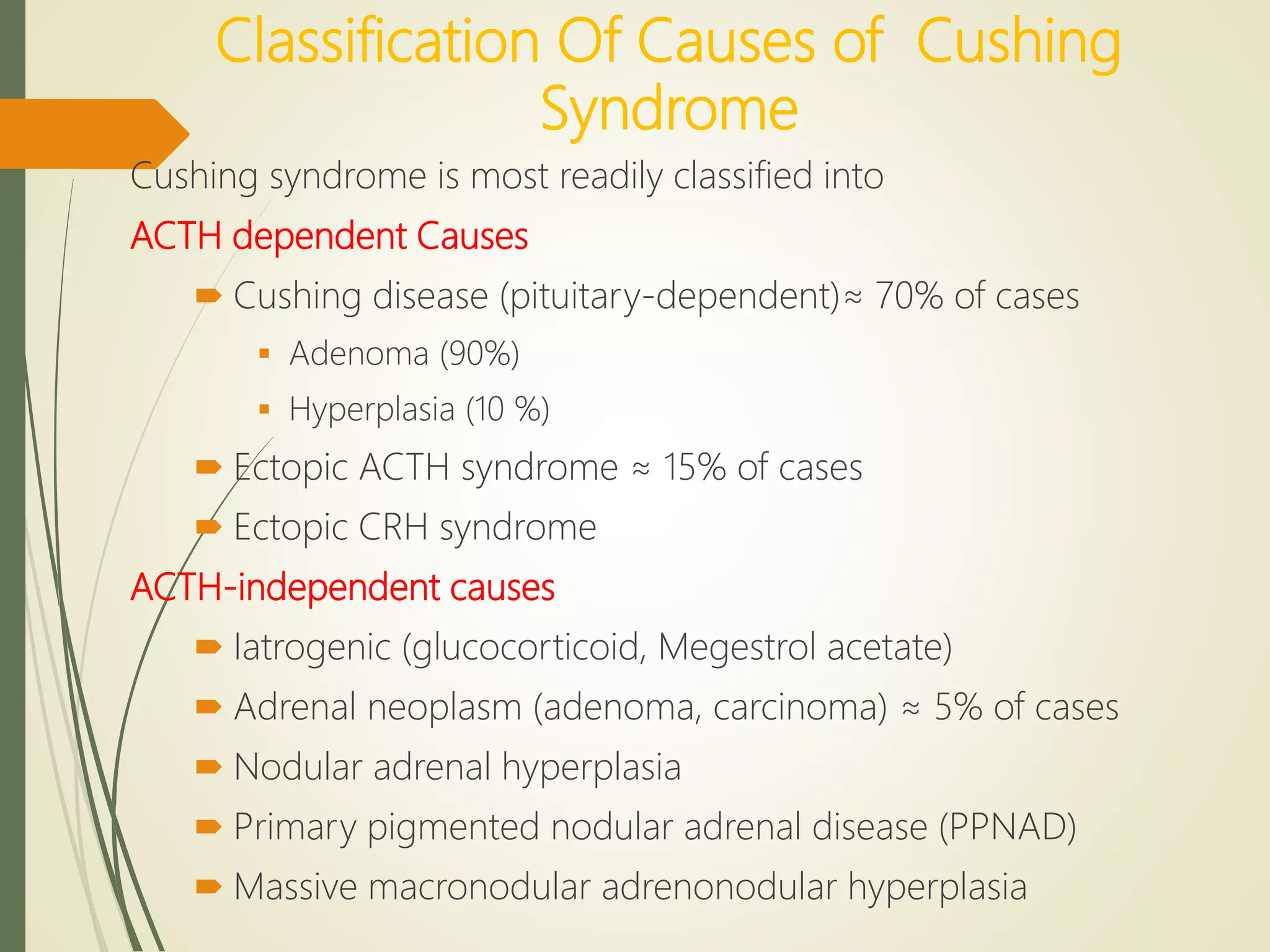
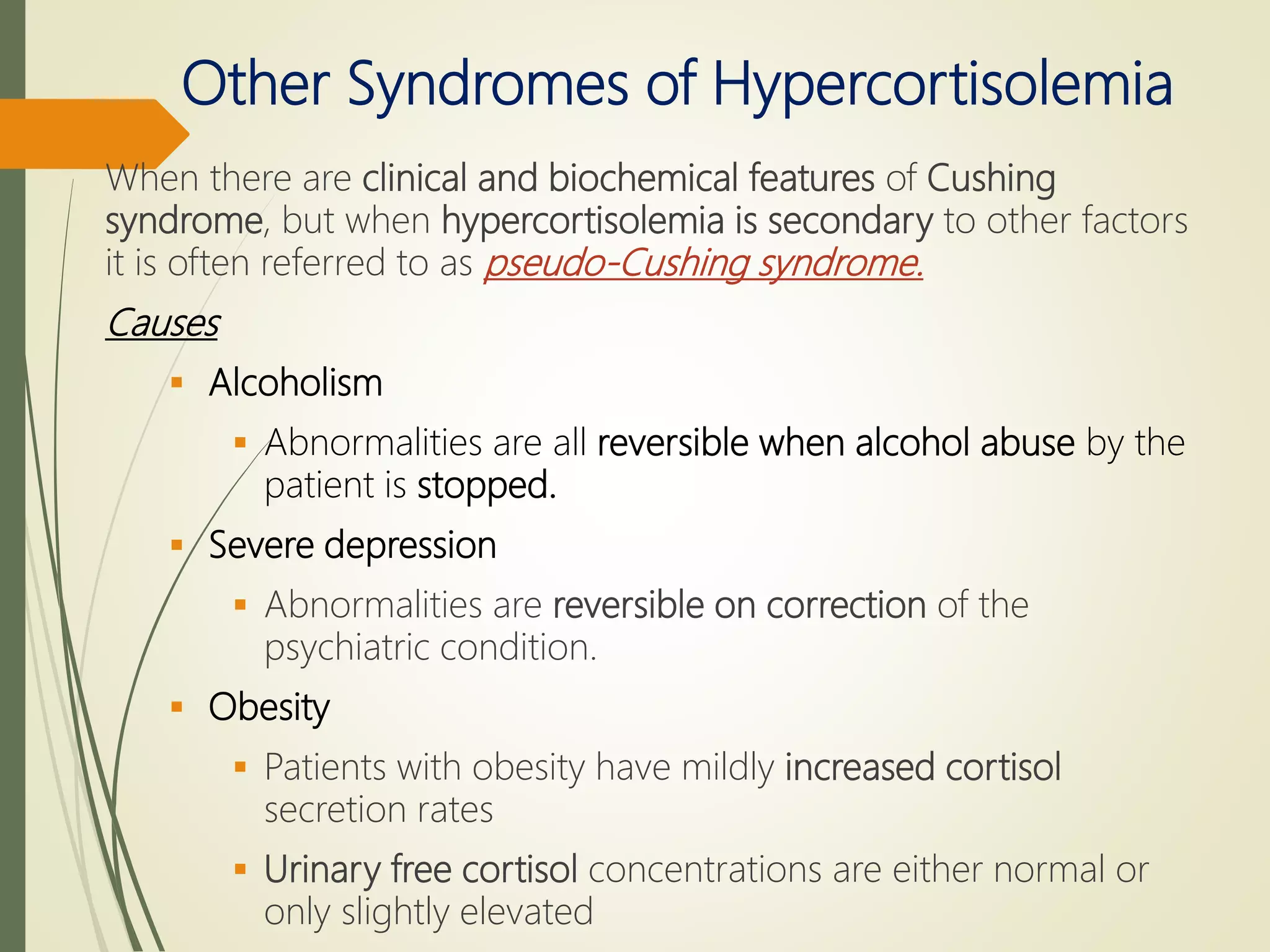
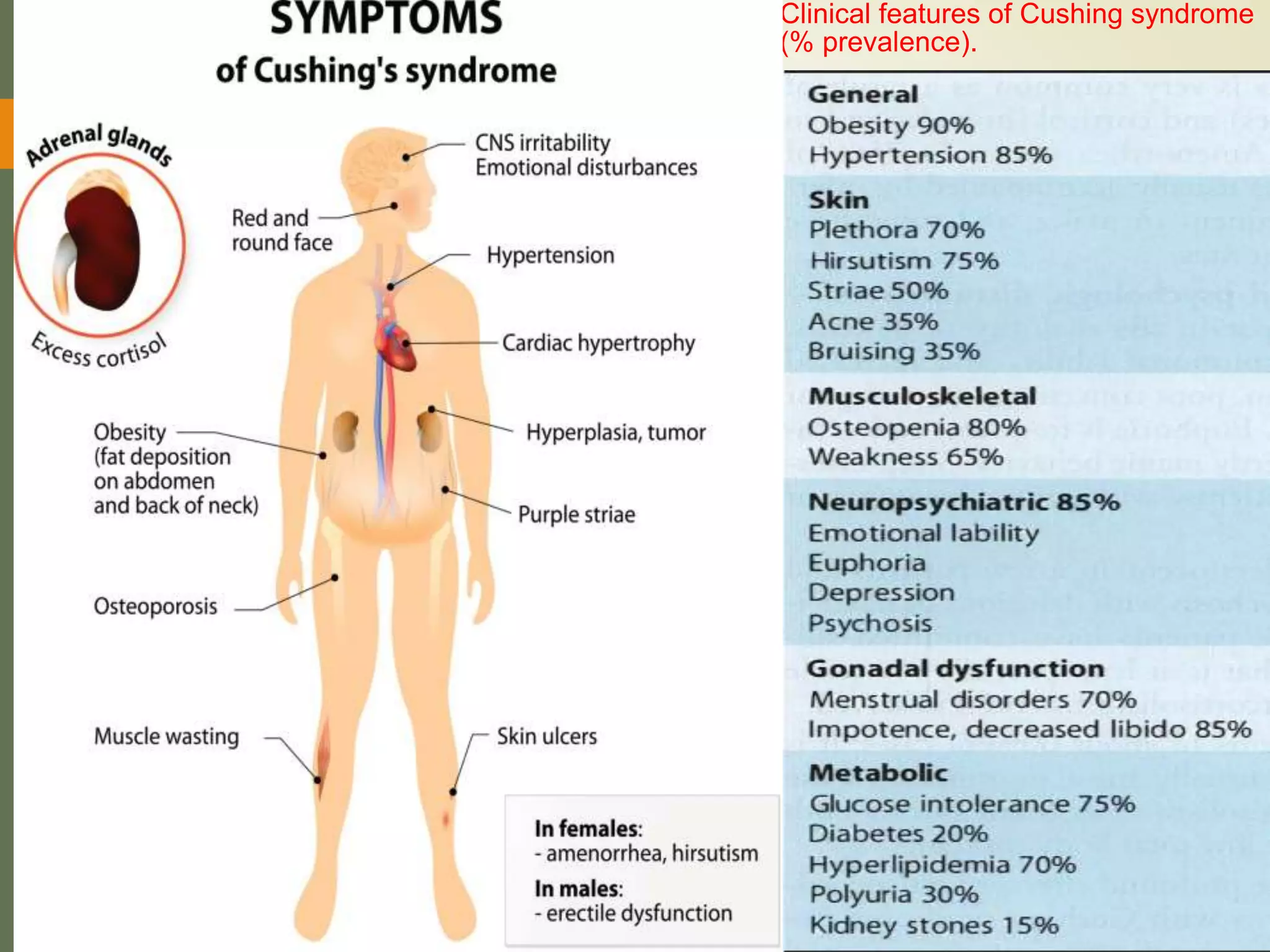
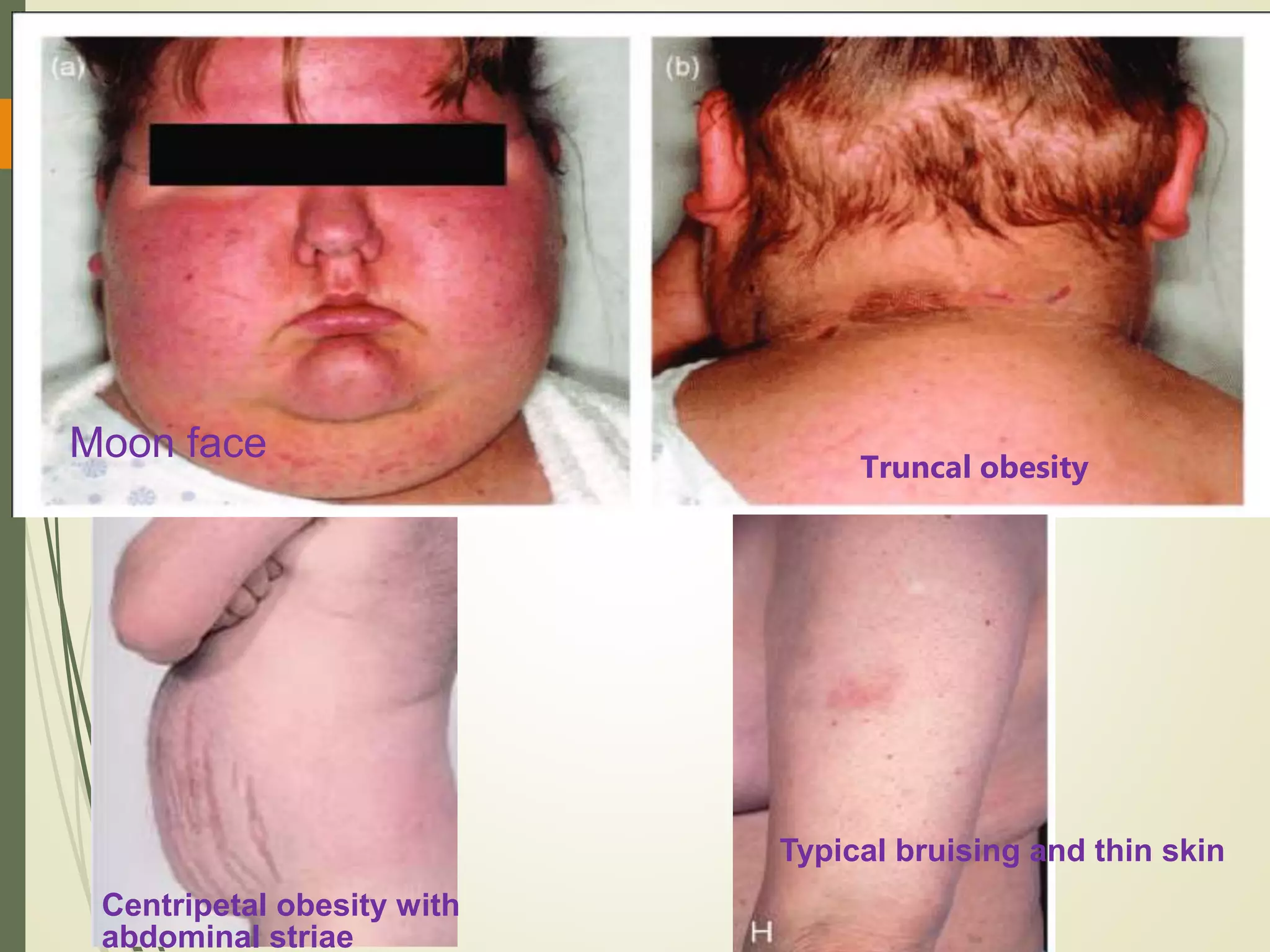
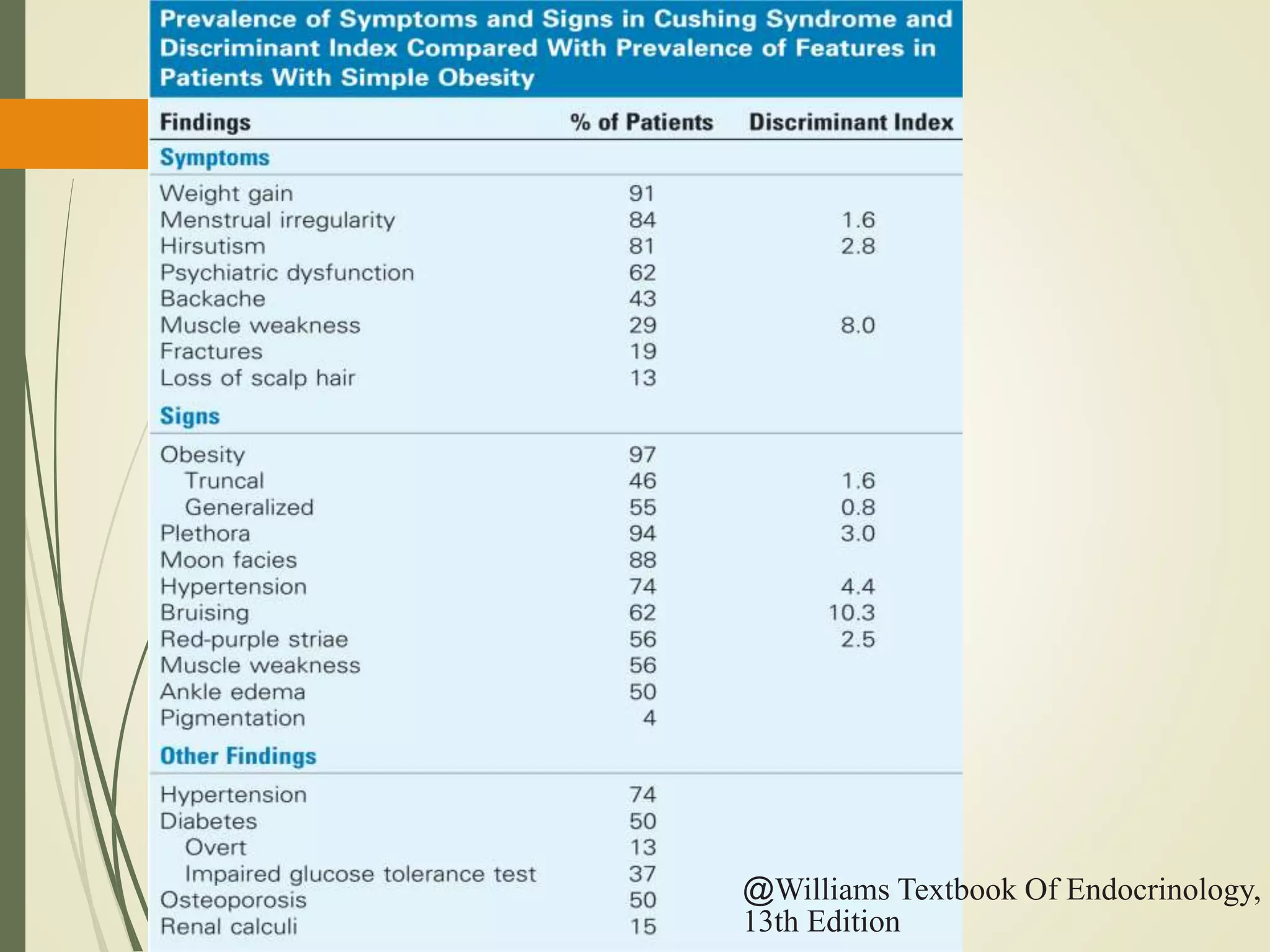
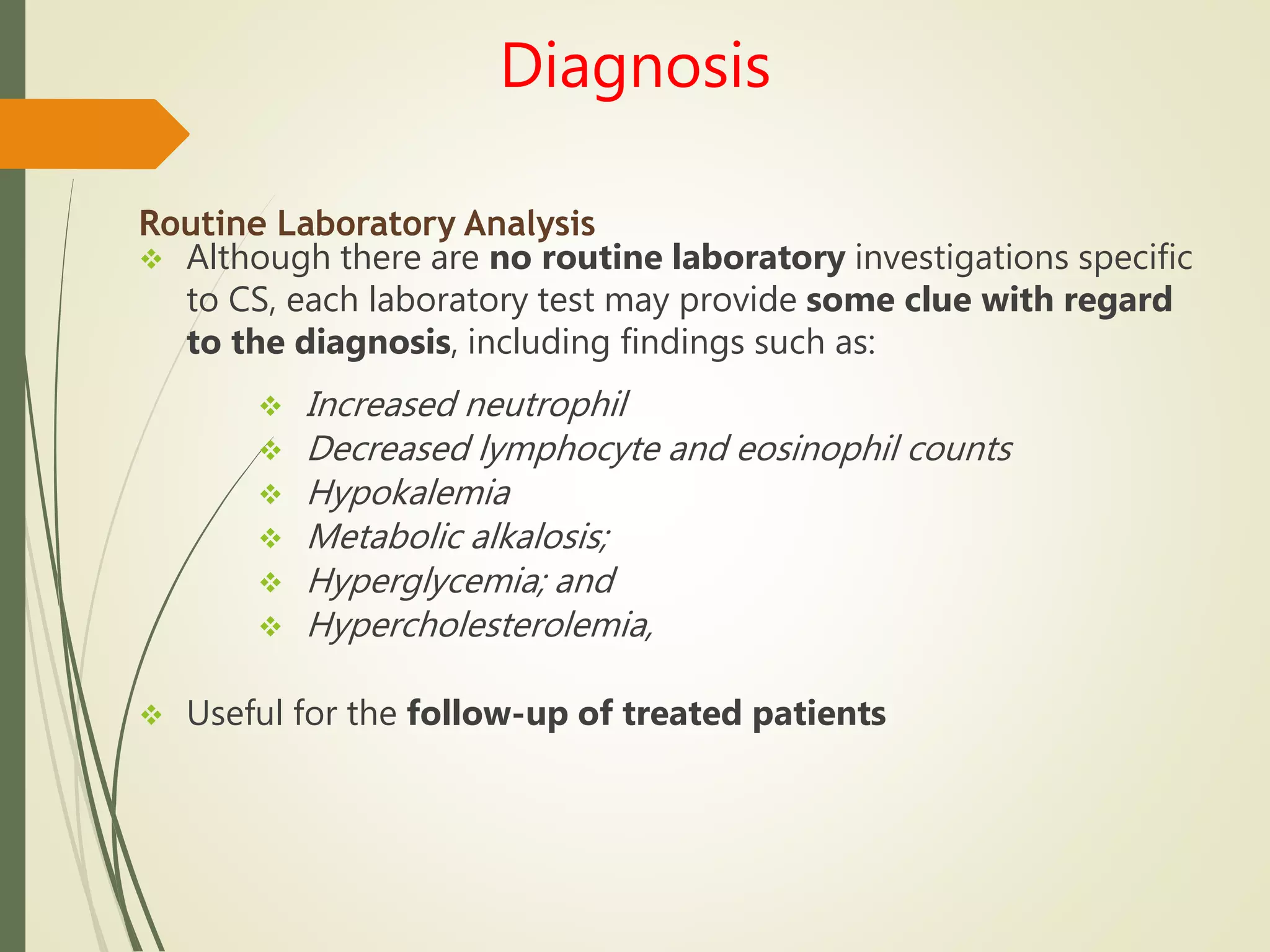
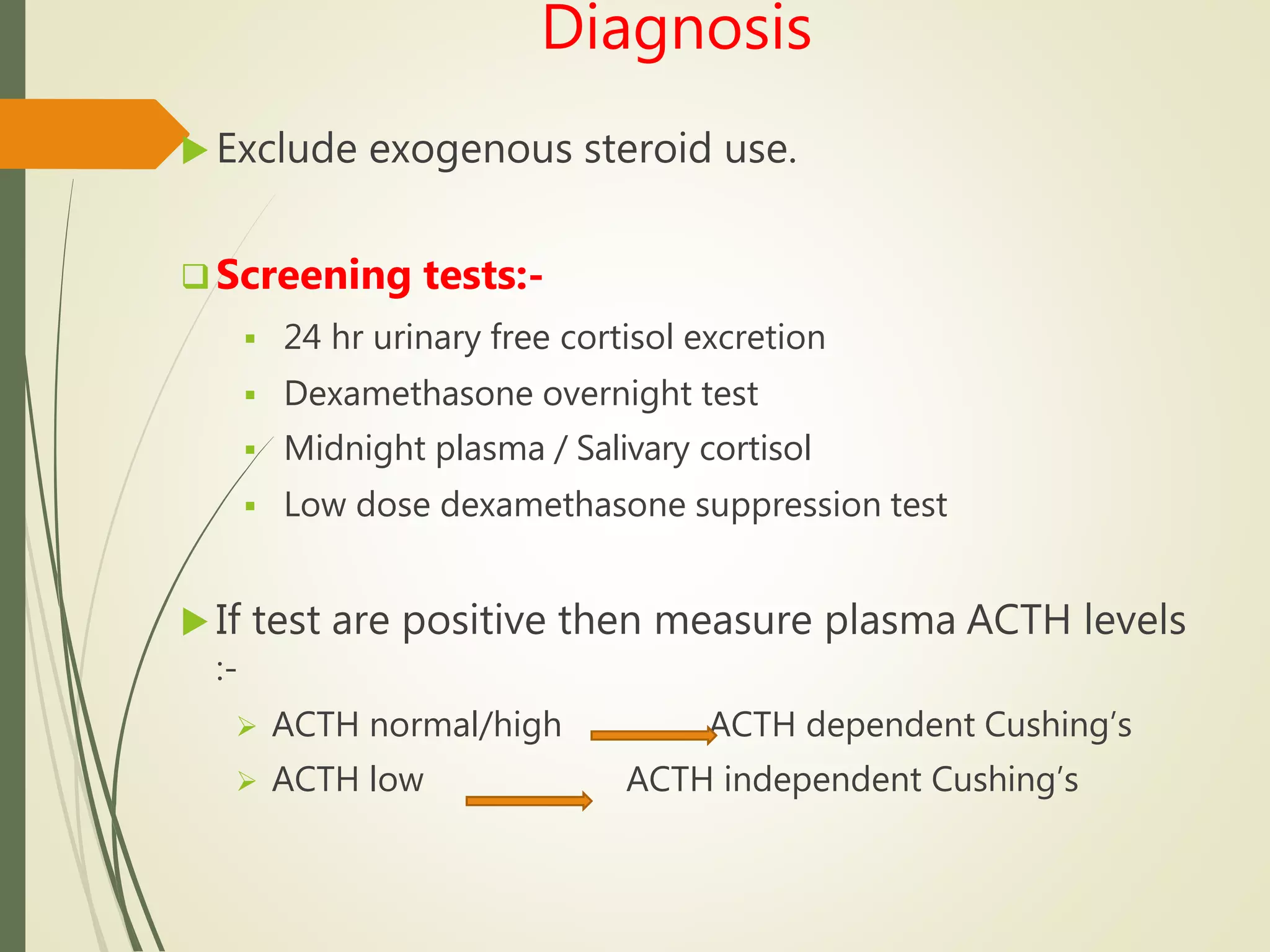
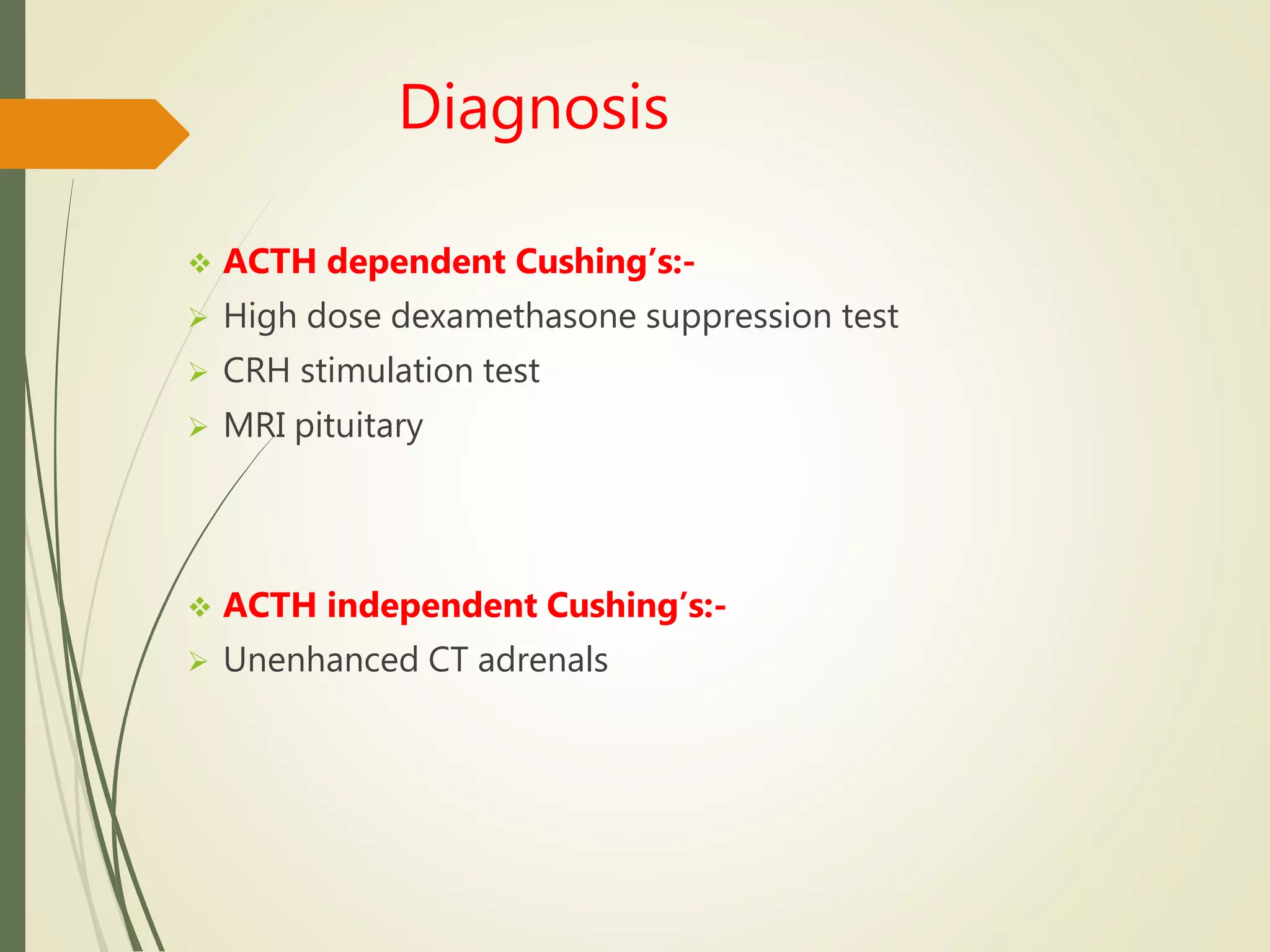
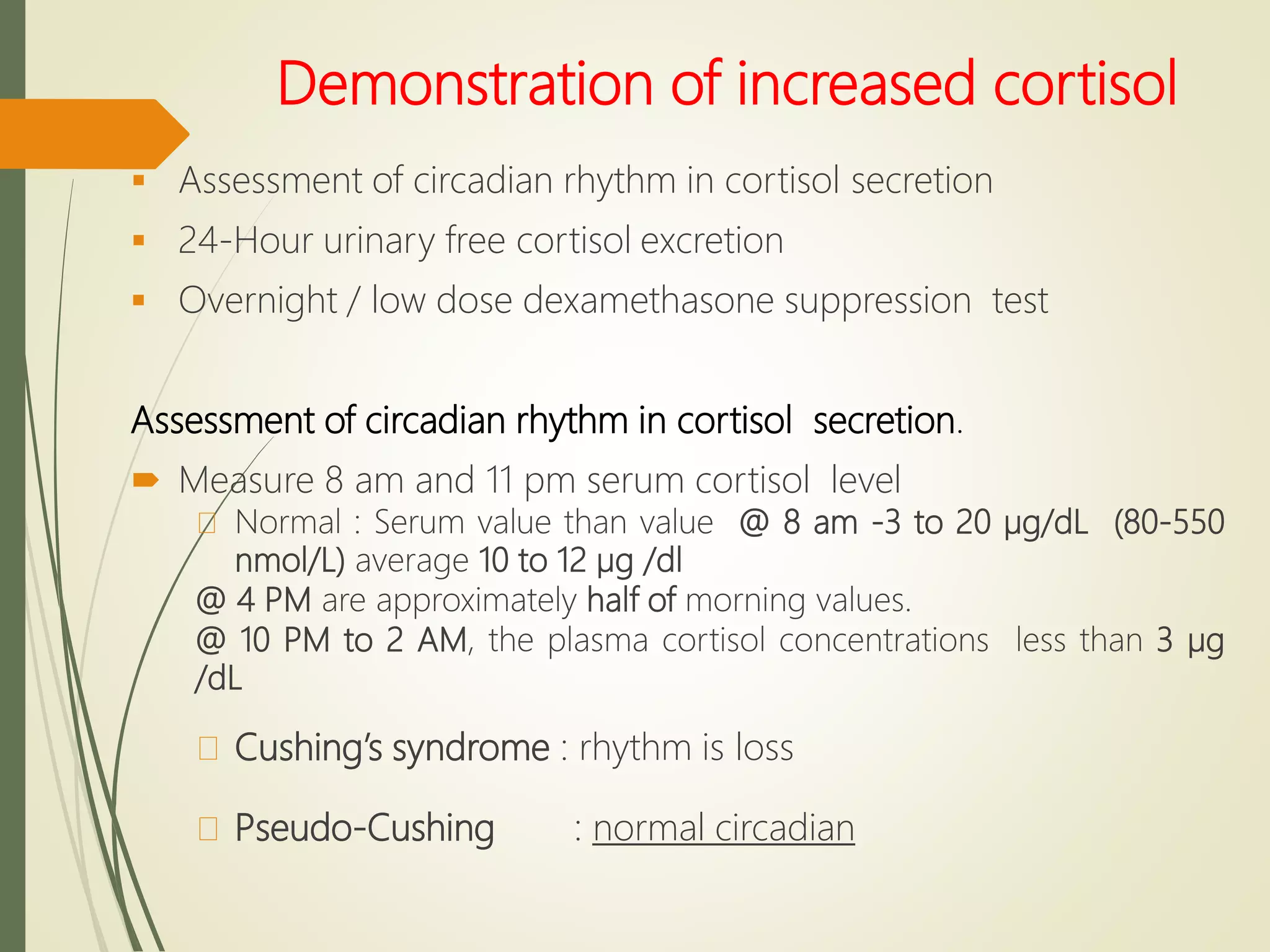
Hyperfunction of the adrenal cortex can cause excess production of mineralocorticoids, glucocorticoids, or androgens. Primary hyperaldosteronism is characterized by autonomous overproduction of aldosterone by the adrenal glands, often leading to hypertension and hypokalemic alkalosis. It can be caused by aldosterone-producing adenomas or bilateral adrenal hyperplasia. Tests like the saline suppression test and adrenal vein sampling are used in the diagnosis. Cushing's syndrome results from prolonged exposure to high levels of cortisol and can be caused by pituitary or adrenal tumors or ectopic ACTH secretion. Signs include central obesity, moon face, and fragile skin






![Differential Diagnosis
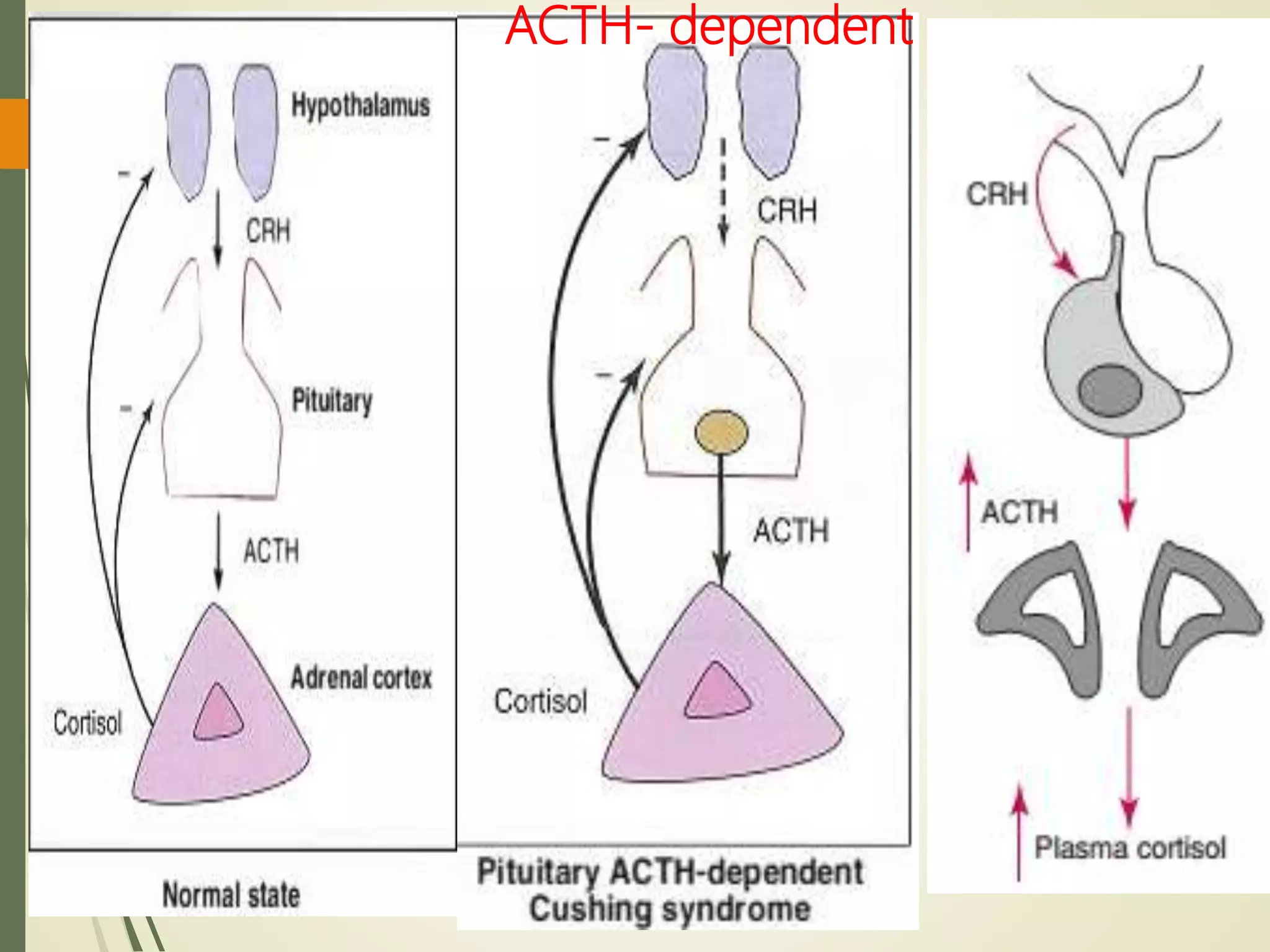
Morning Plasma ACTH
Differentiates ACTH-dependent from
ACTH-independent causes.
In Cushing disease
50% of patients have a 9 AM ACTH
level within the normal reference
range (2 to
11 pmol/L [9 to 52 pg/mL]);
in the remainder- modestly
elevated.
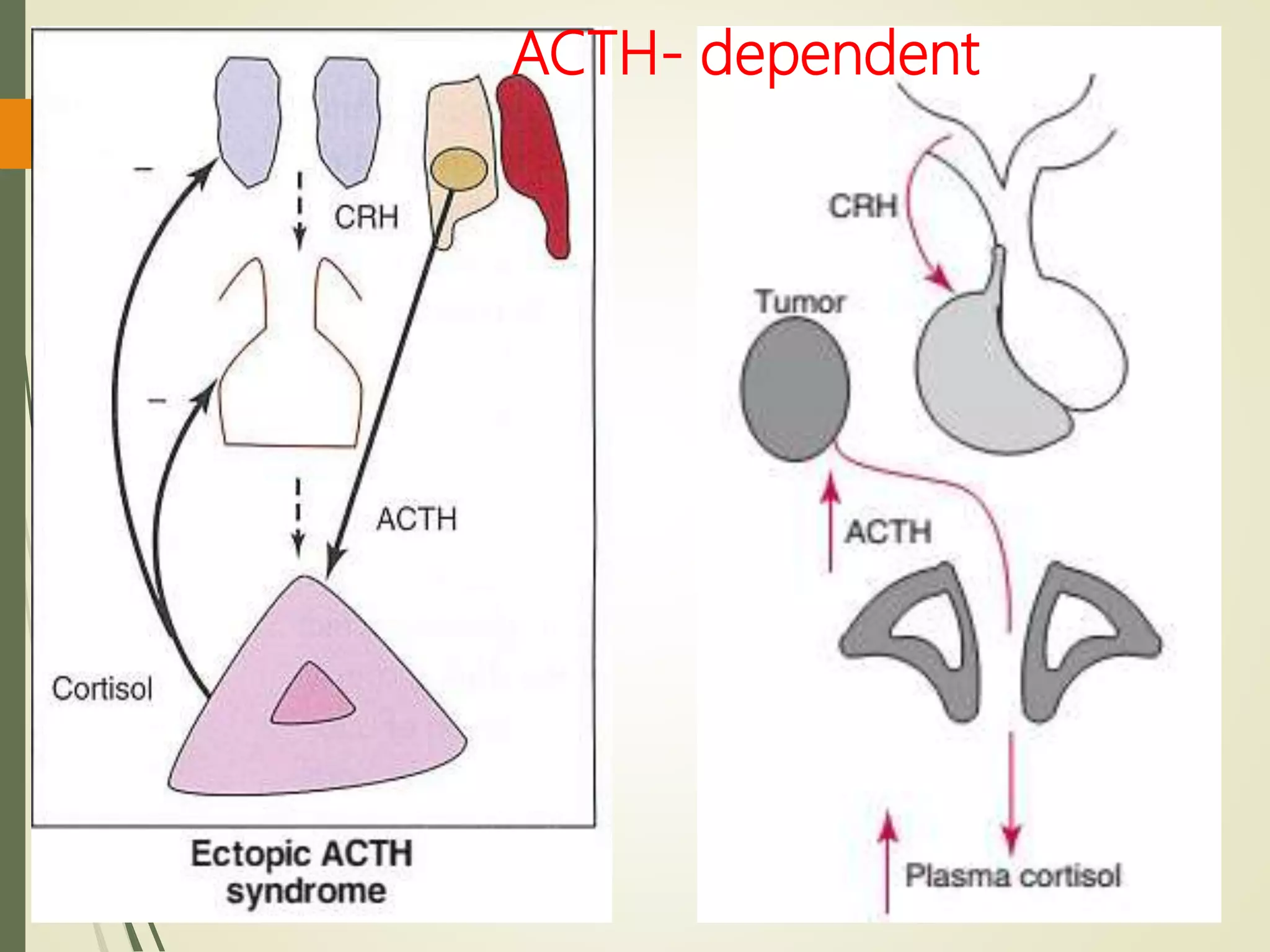
In the ectopic ACTH syndrome
ACTH levels are high (usually >20
pmol/L [>90 pg/mL]);
Overlap values are seen in
Cushing disease in 30% of cases
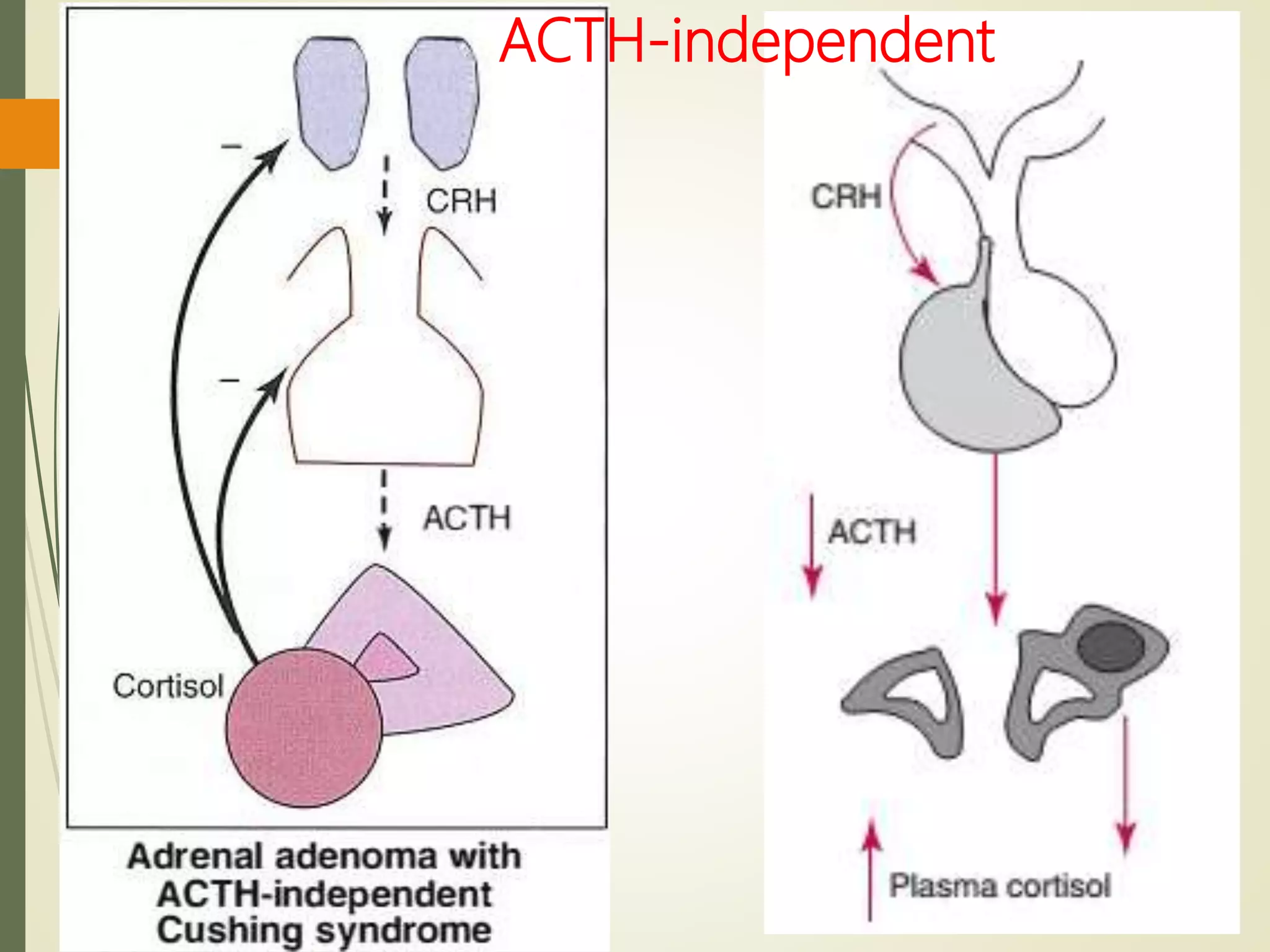
In patients with adrenal tumors, plasma
ACTH is invariably undetectable (<1
pmol/L).](https://image.slidesharecdn.com/hyperfunctionofadrenal-210420155319/75/Hyper-function-of-adrenal-40-2048.jpg)