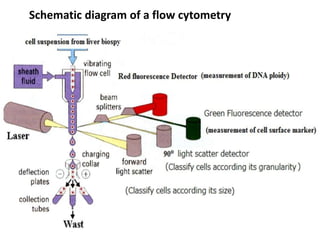
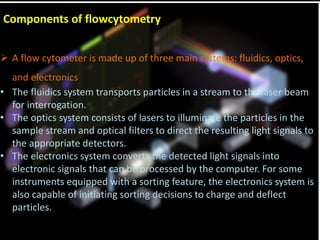
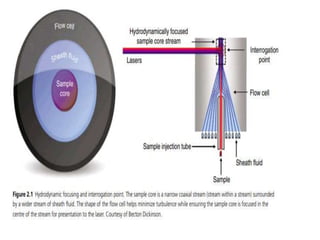
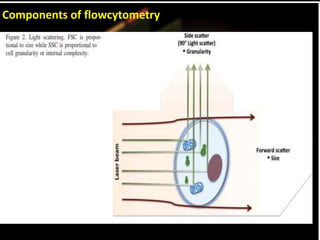
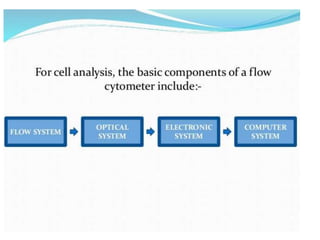
Flow cytometry is a laser-based technique used to analyze physical and chemical properties of cells/particles as they flow in a fluid stream through a laser beam. It allows for rapid, multiparameter analysis of individual cells. The key components of a flow cytometer are fluidics, optics, and electronics systems. Lasers illuminate cells and light is scattered and detected to analyze properties like size, granularity, and fluorescence. Applications in food microbiology include quantifying bacteria in foods and beverages, monitoring dairy starters, and studying probiotics under stress conditions.