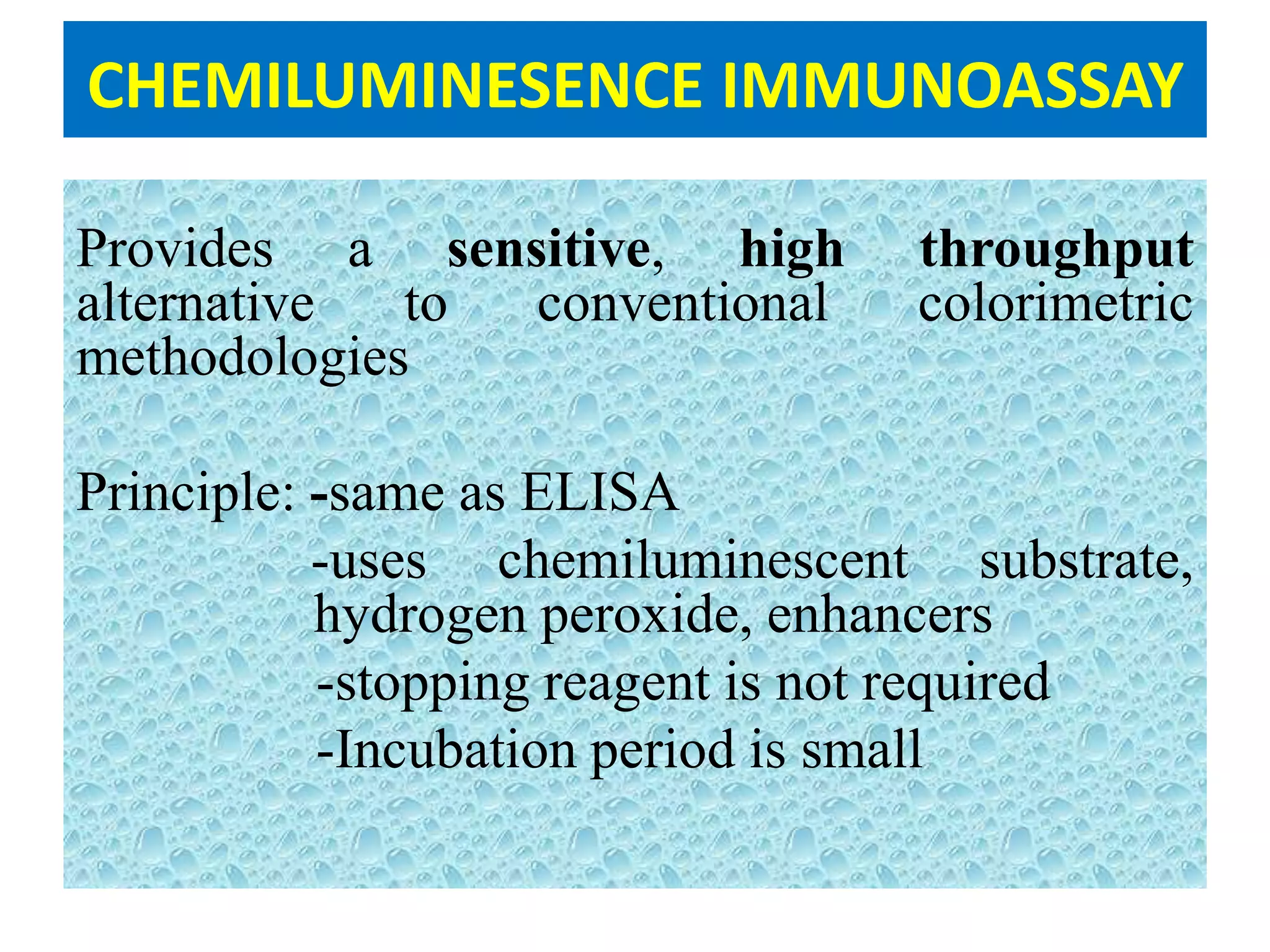
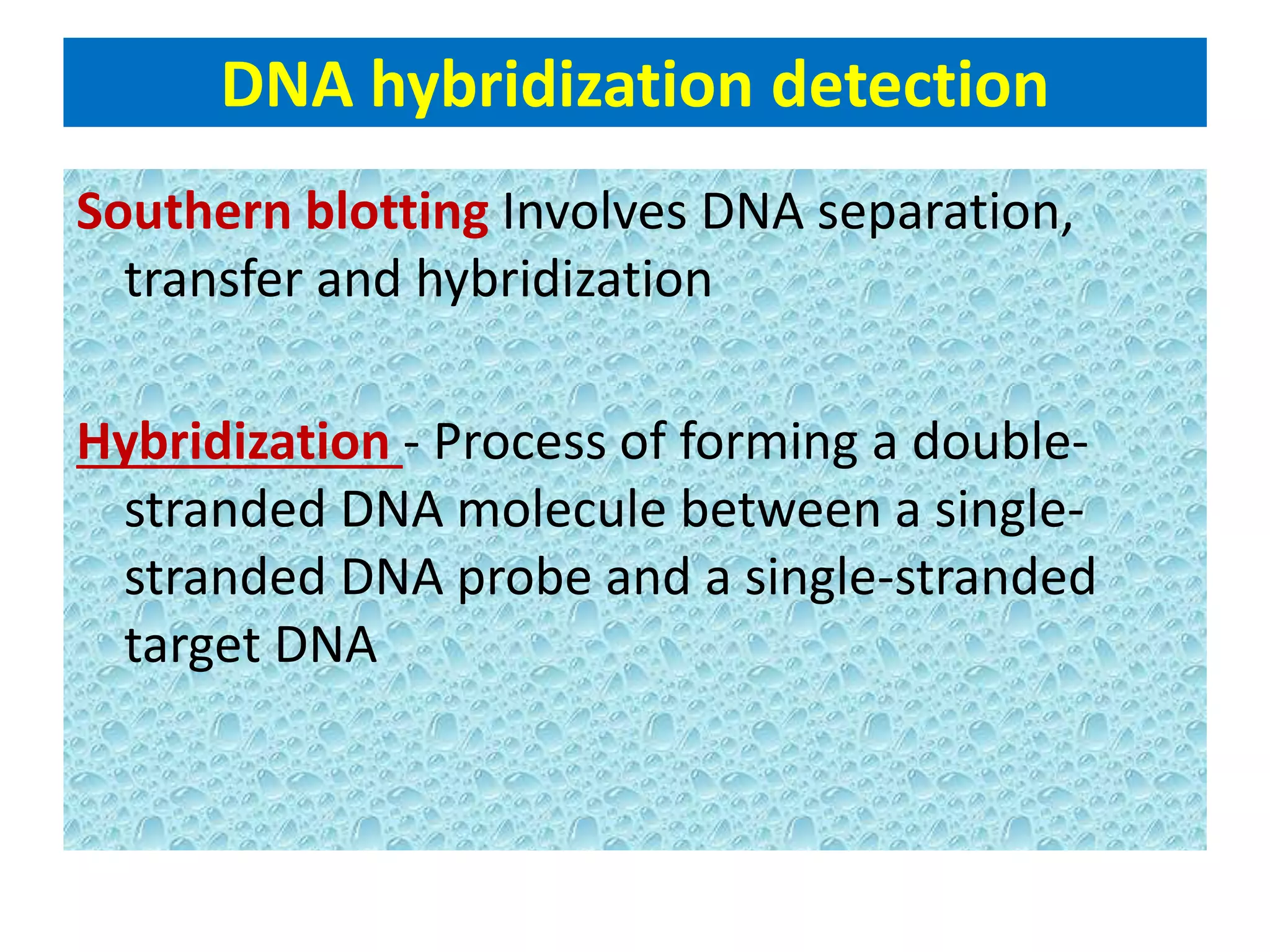
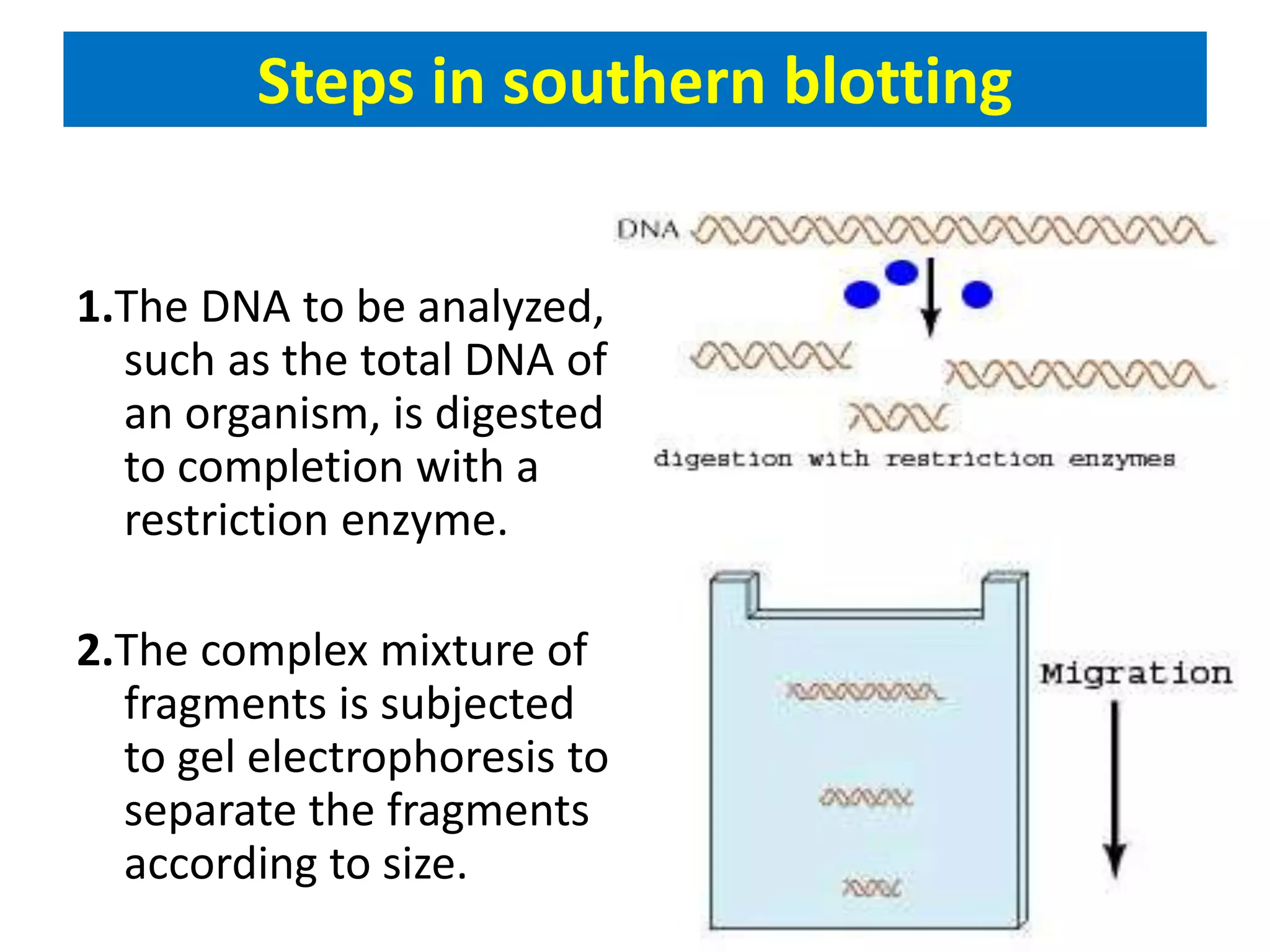
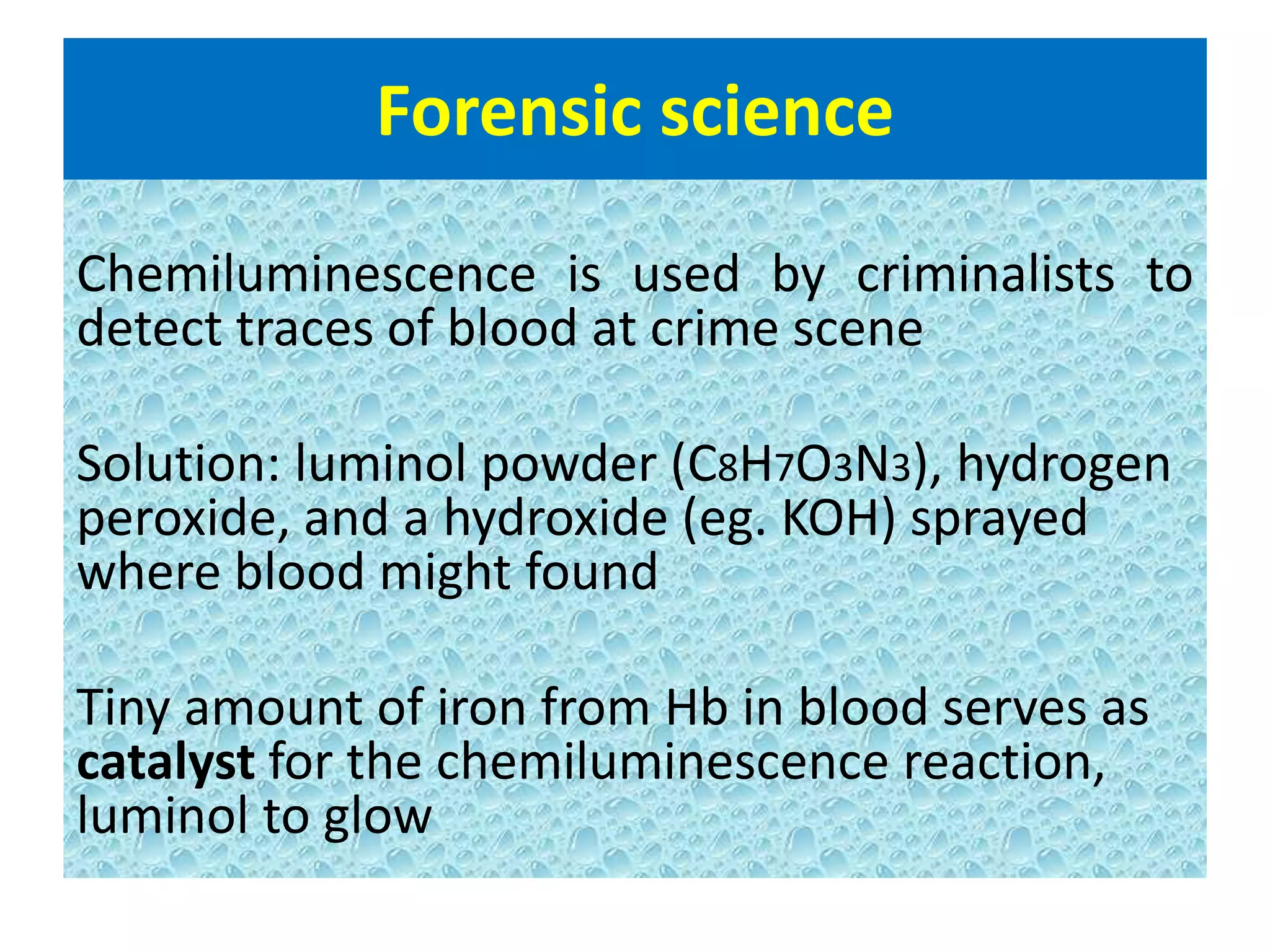
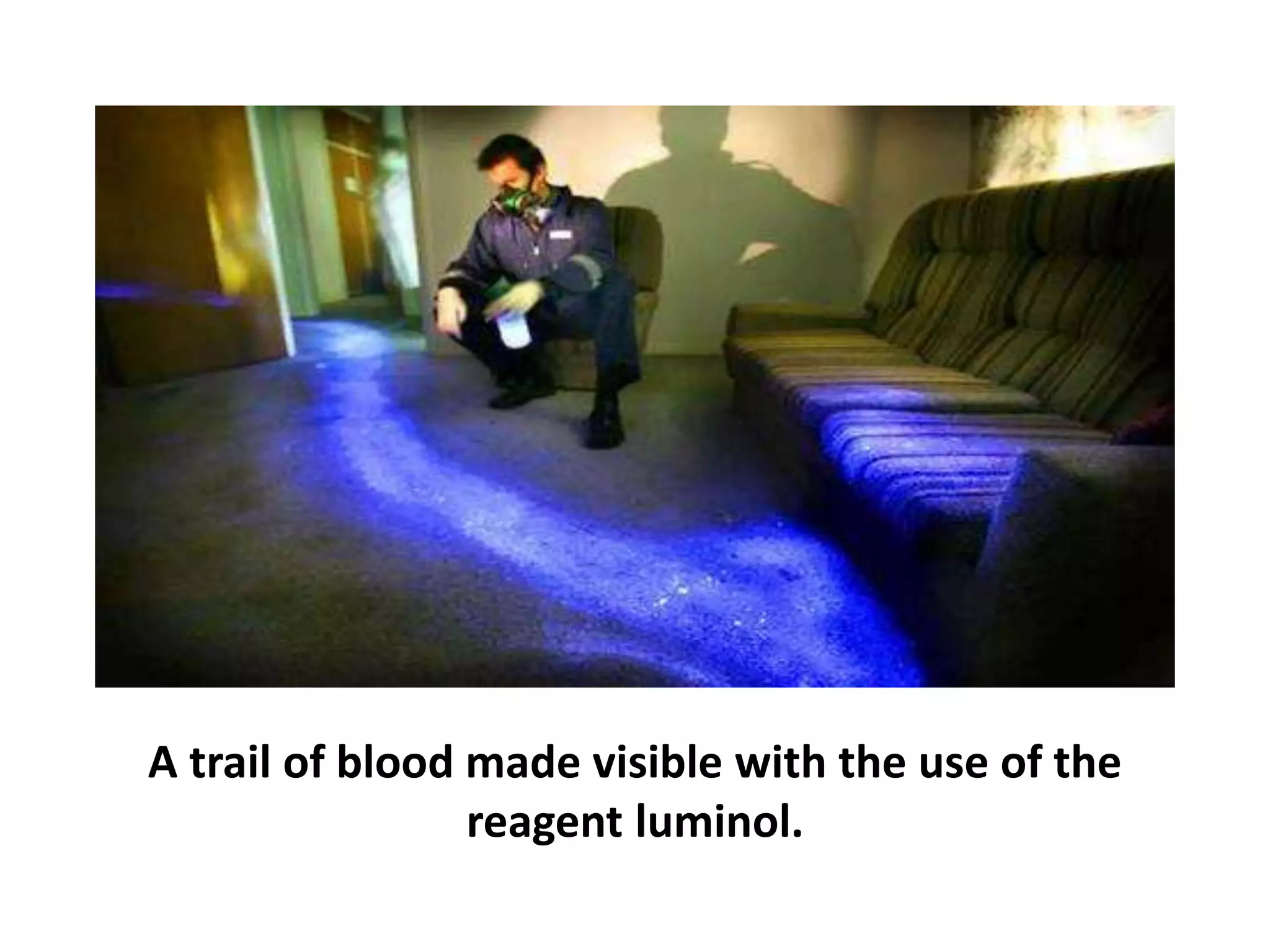
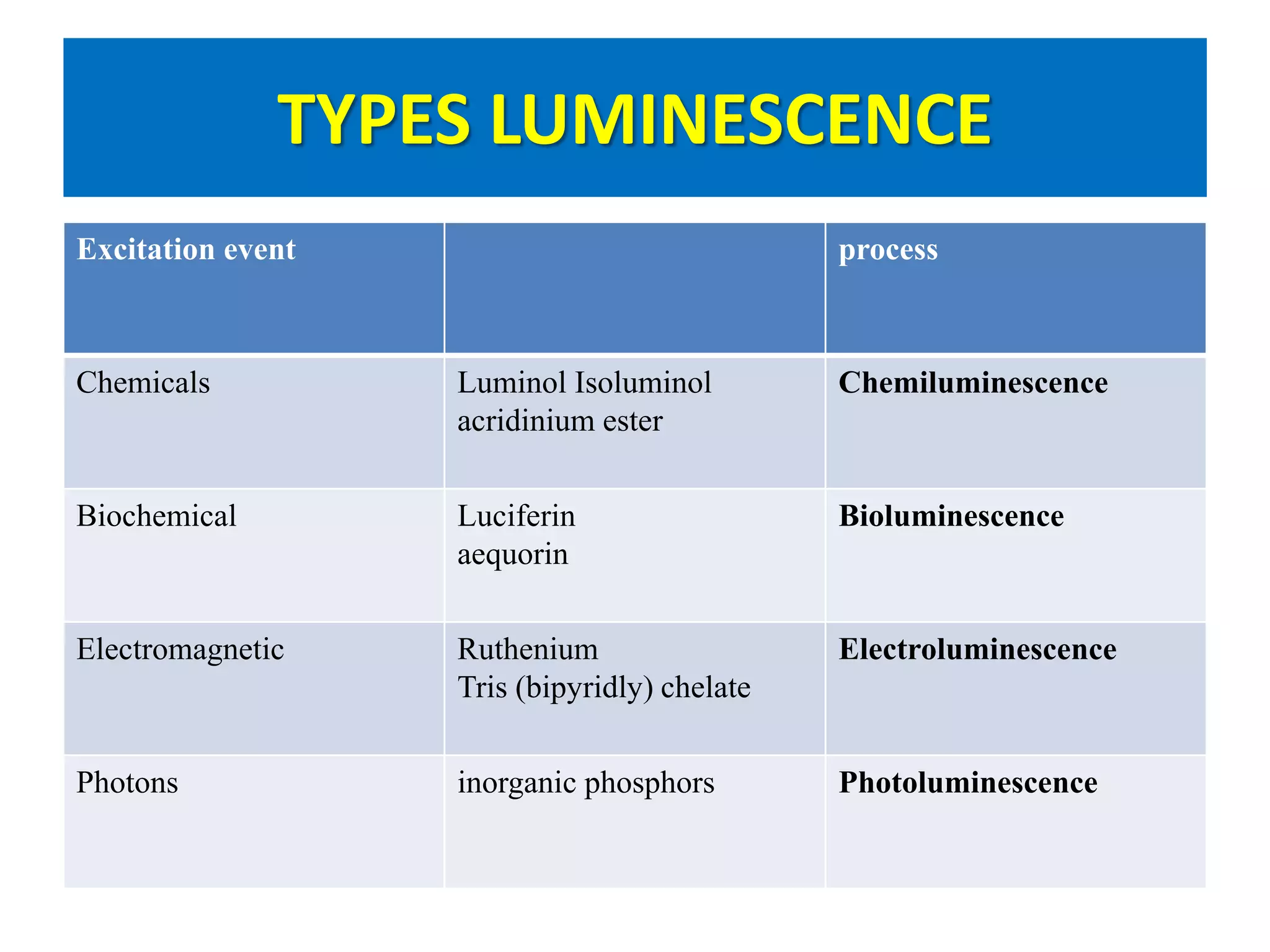
This document discusses chemiluminescence, which is the emission of light from a chemical reaction without significant heat production. It describes how chemiluminescence occurs when a reactant kicks an electron in an atom to an excited state, and the electron then returns to the ground state emitting a photon of light. Common examples of chemiluminescent reactions involve luminol, isoluminol, and luciferin. The document outlines applications of chemiluminescence including immunoassays, DNA hybridization detection, western blotting, forensic analysis to detect blood, and food analysis to detect pesticides. It also notes some limitations such as light leaks and high intensity light saturation effects.


![CHEMILUMINESCENCE
Emission of light with limited emission of heat
(luminescence), as the result of a chemical
reaction.
[A] + [B] → [◊] → [Products] + light
[A], [B]: reactants
[◊]: excited intermediate](https://image.slidesharecdn.com/chemiluminescence-150401111426-conversion-gate01/75/Chemiluminescence-4-2048.jpg)
![For example, if [A] is luminol and [B] is hydrogen
peroxide in the presence of a suitable catalyst we
have:
luminol + H2O2 →3-APA[◊] →3-APA + light
Where:
3-APA is 3-aminophthalate
3-APA[◊] is the excited state producing light as
it decays to a lower energy level.](https://image.slidesharecdn.com/chemiluminescence-150401111426-conversion-gate01/75/Chemiluminescence-5-2048.jpg)