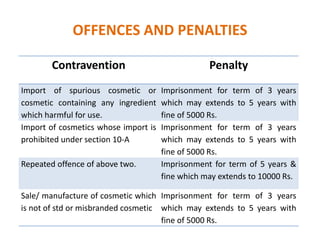
The document provides an overview of cosmetics regulation under the Drug and Cosmetic Act, 1940 in India, detailing the process for obtaining manufacturing licenses, categorizing cosmetics, and establishing quality standards. It outlines the requirements for production facilities, labeling, and testing of cosmetics, as well as penalties for violations, including the import and sale of unsafe or misbranded products. Additionally, it mentions provisions for loan licensing and lists prohibited cosmetics to ensure consumer safety.