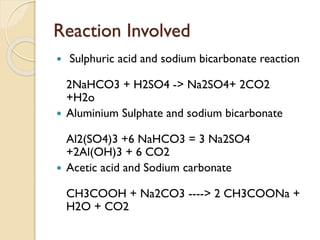
The document presents an innovative guide for developing a fire extinguisher, detailing types of extinguishers, improvements in materials and design, and the chemistry behind their operation. It highlights the specific reactions involved, provides calculations for enthalpy and entropy, and discusses future prospects for the manufacturing and design of fire extinguishers. The conclusion emphasizes the project's objective of creating a cost-effective, easy-to-use extinguisher model while acknowledging the support from Thadomal Shahani Engineering College.