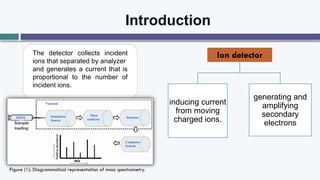
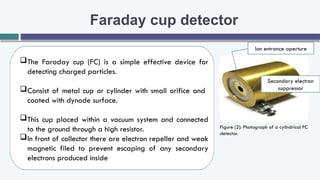
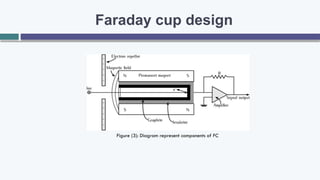
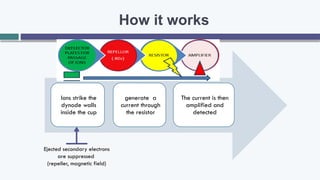
The document provides an overview of the Faraday cup (FC) detector, a device for ion detection that generates a current from moving charged ions, enabling accurate measurements of ion abundance. It details the FC design, efficiency, applications in isotope ratio mass spectrometry, quantitative analysis, and instrument calibration, noting its simplicity and reliability. However, it also mentions limitations such as lower sensitivity and slower response time compared to other detectors.