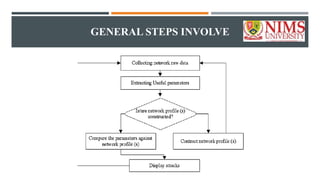
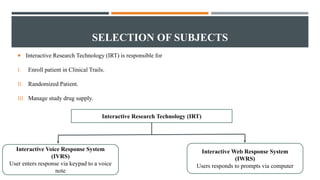
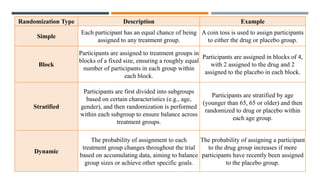
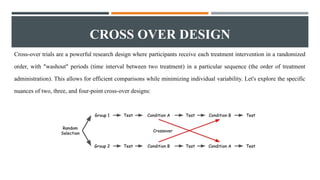
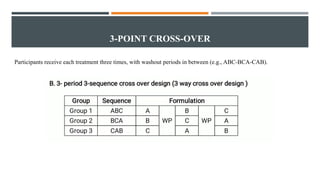
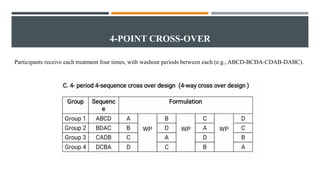
The document outlines general research methodology in the field of pharmaceutics, detailing key concepts such as research objectives, types of studies, and strategies to mitigate errors and bias. It describes various research designs, including descriptive and analytical studies, and emphasizes the importance of ethical considerations, rigorous data collection, and clear reporting in conducting research. Additionally, it explores the significance of literature reviews and provides guidance on study design, specifically observational and experimental methods.