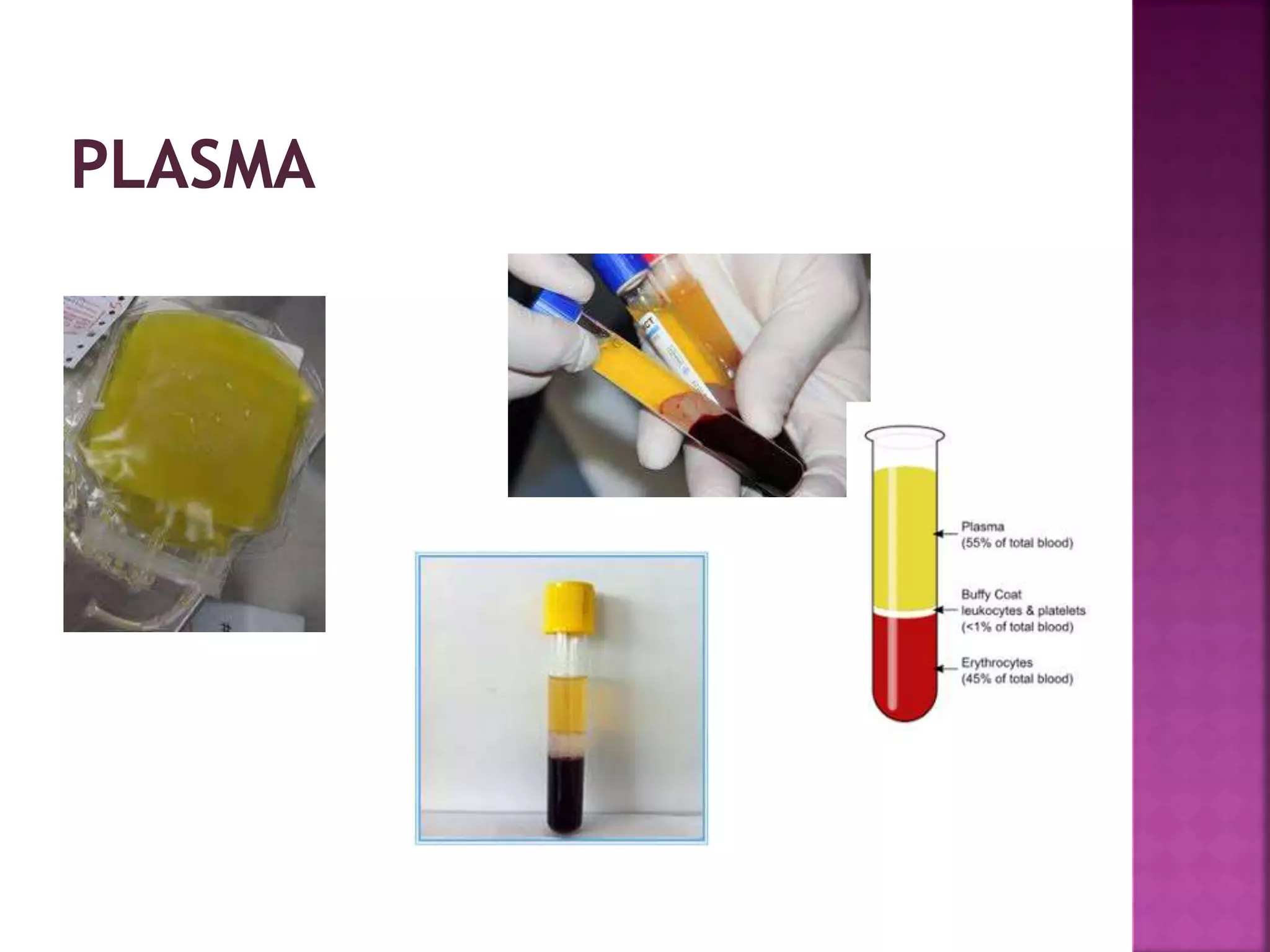
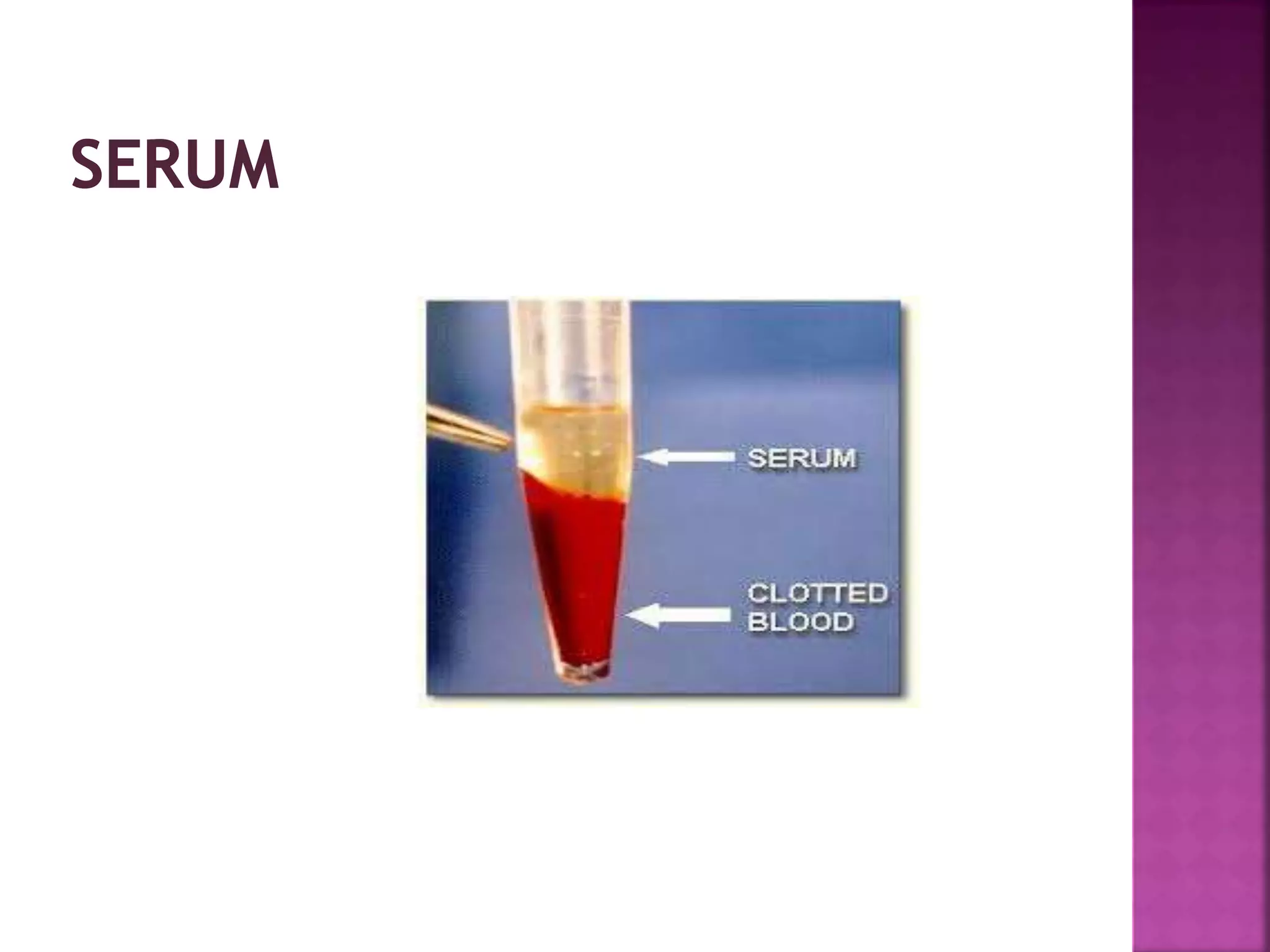
This document discusses bioanalytical methods for quantitative analysis of compositions in complex biological samples. It describes various sample preparation techniques like protein precipitation, liquid-liquid extraction, and solid phase extraction that are used to extract analytes from matrices and remove interfering compounds. It also discusses different types of biological samples like plasma, serum, whole blood, urine, and saliva that are used for analysis and the challenges associated with each matrix. Maintaining sample integrity from collection to analysis is important for obtaining reliable results.


![ The separation of the component is often
performed prior to the analysis.
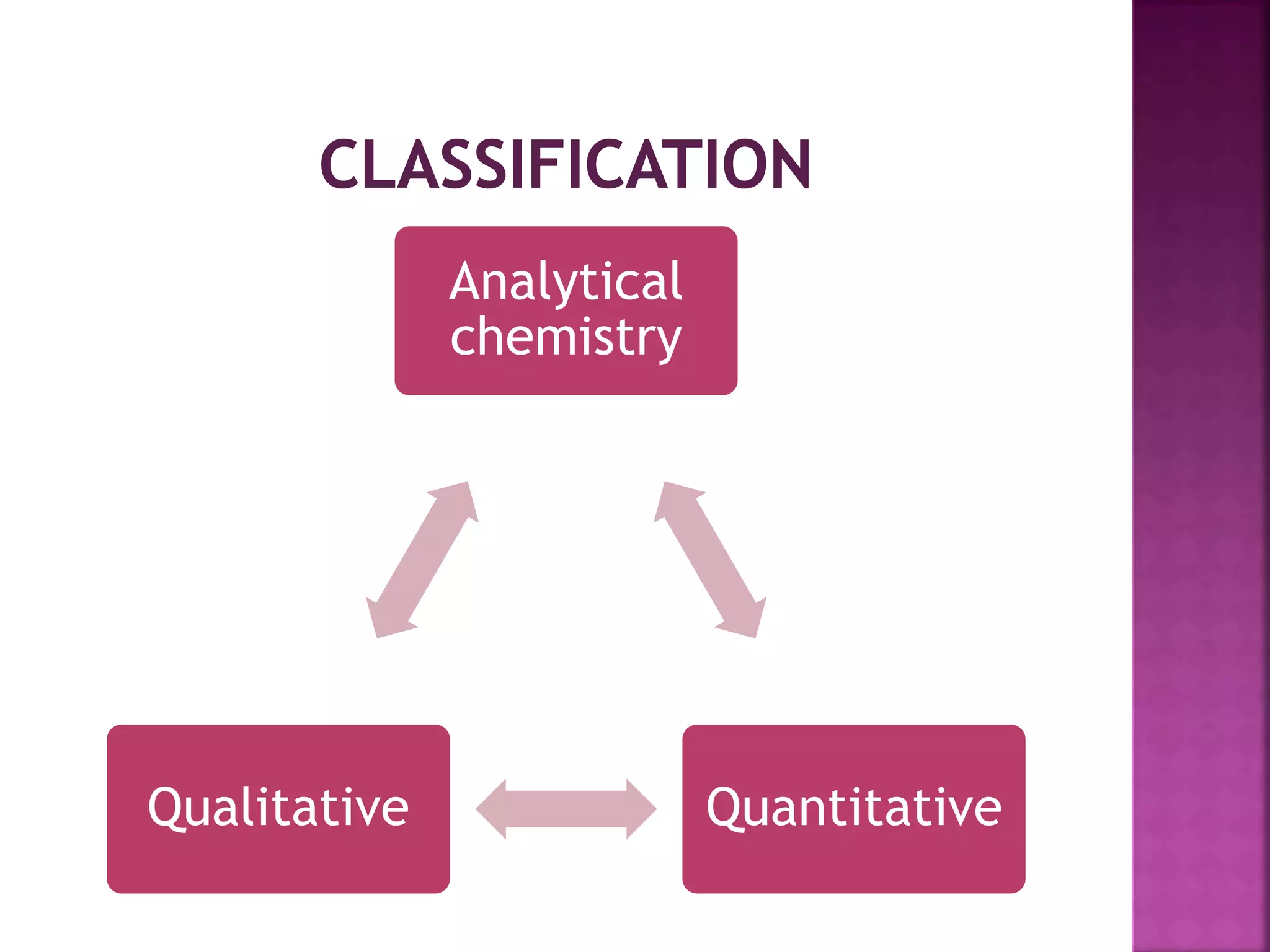
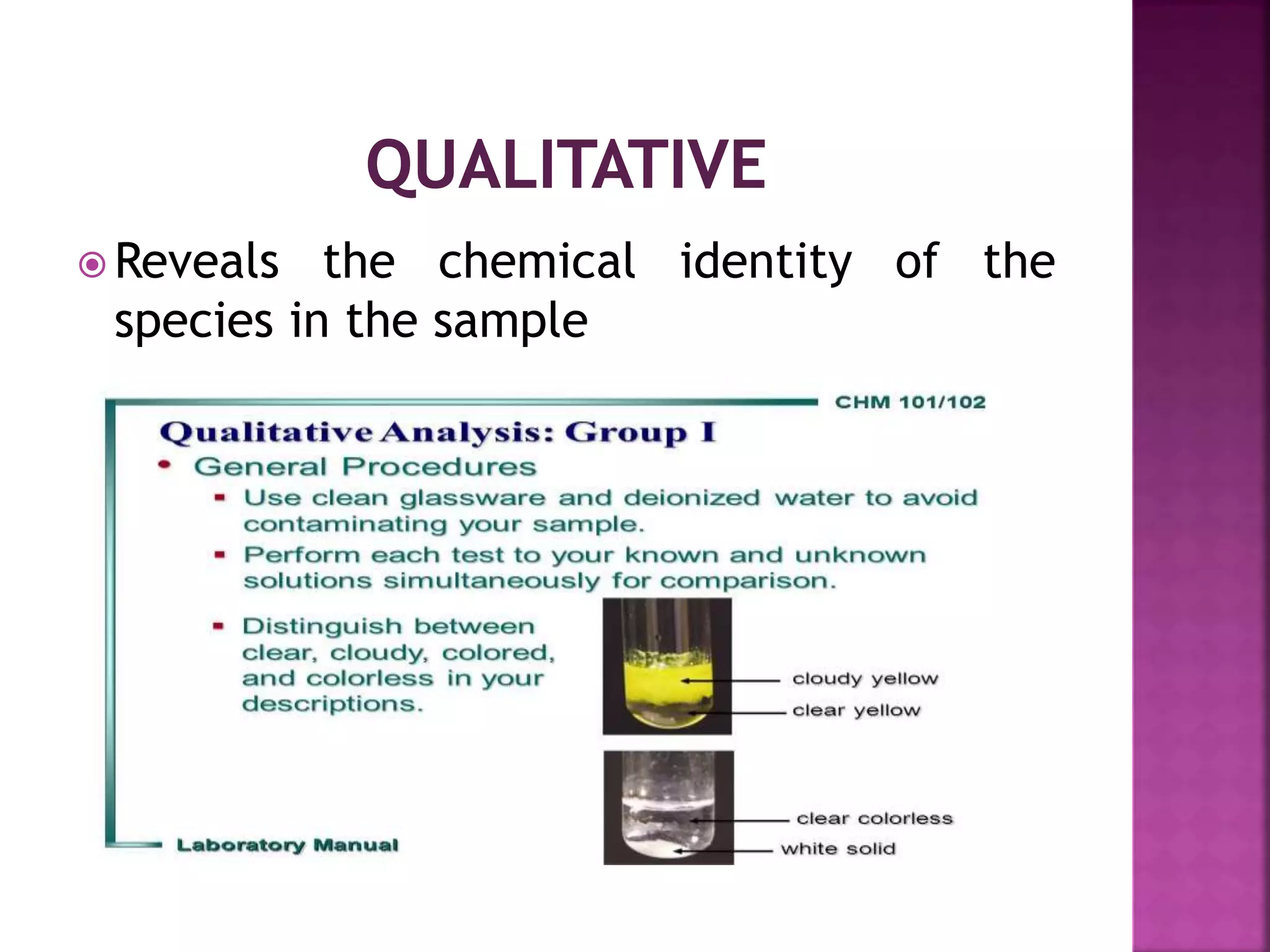
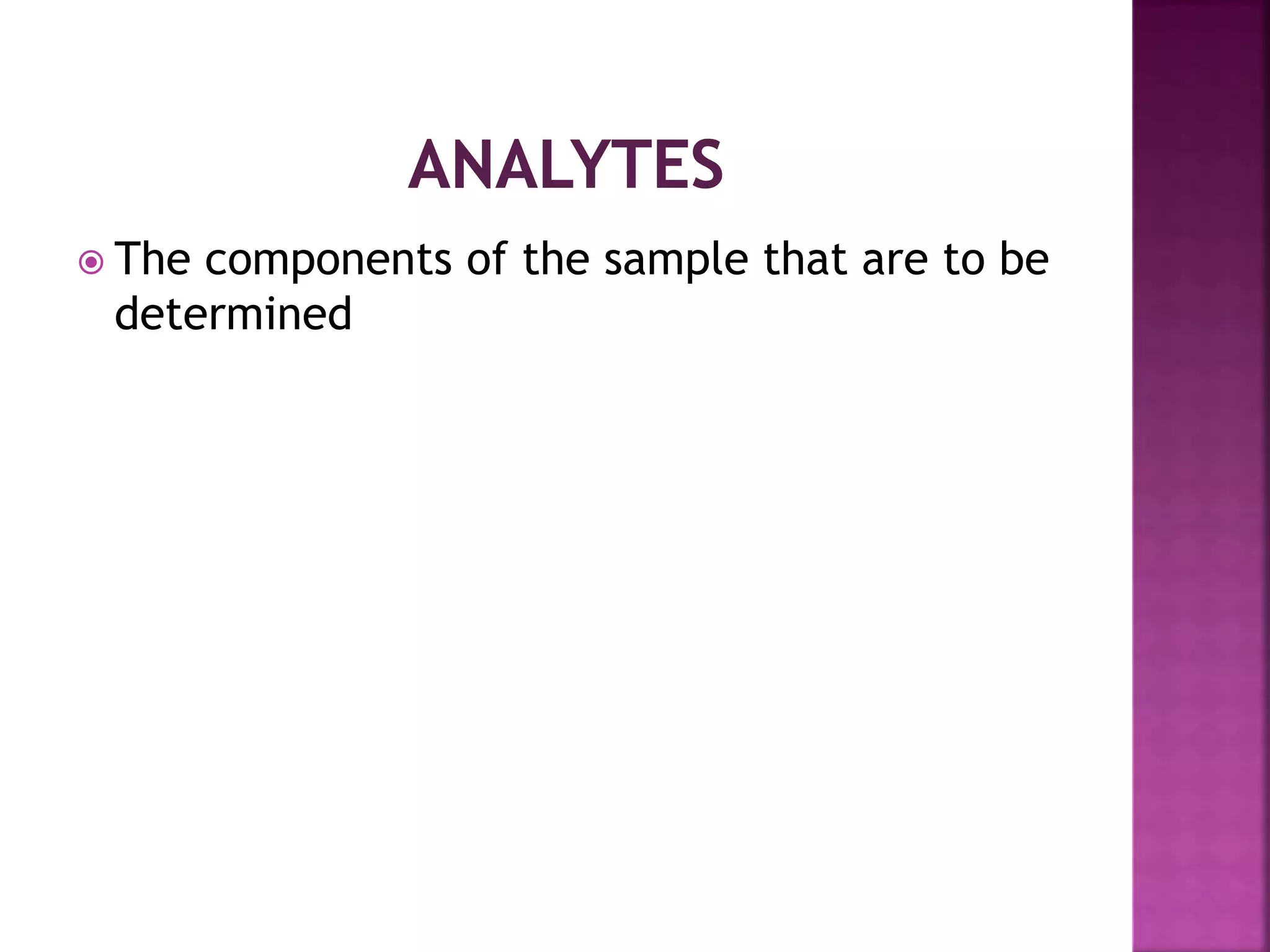
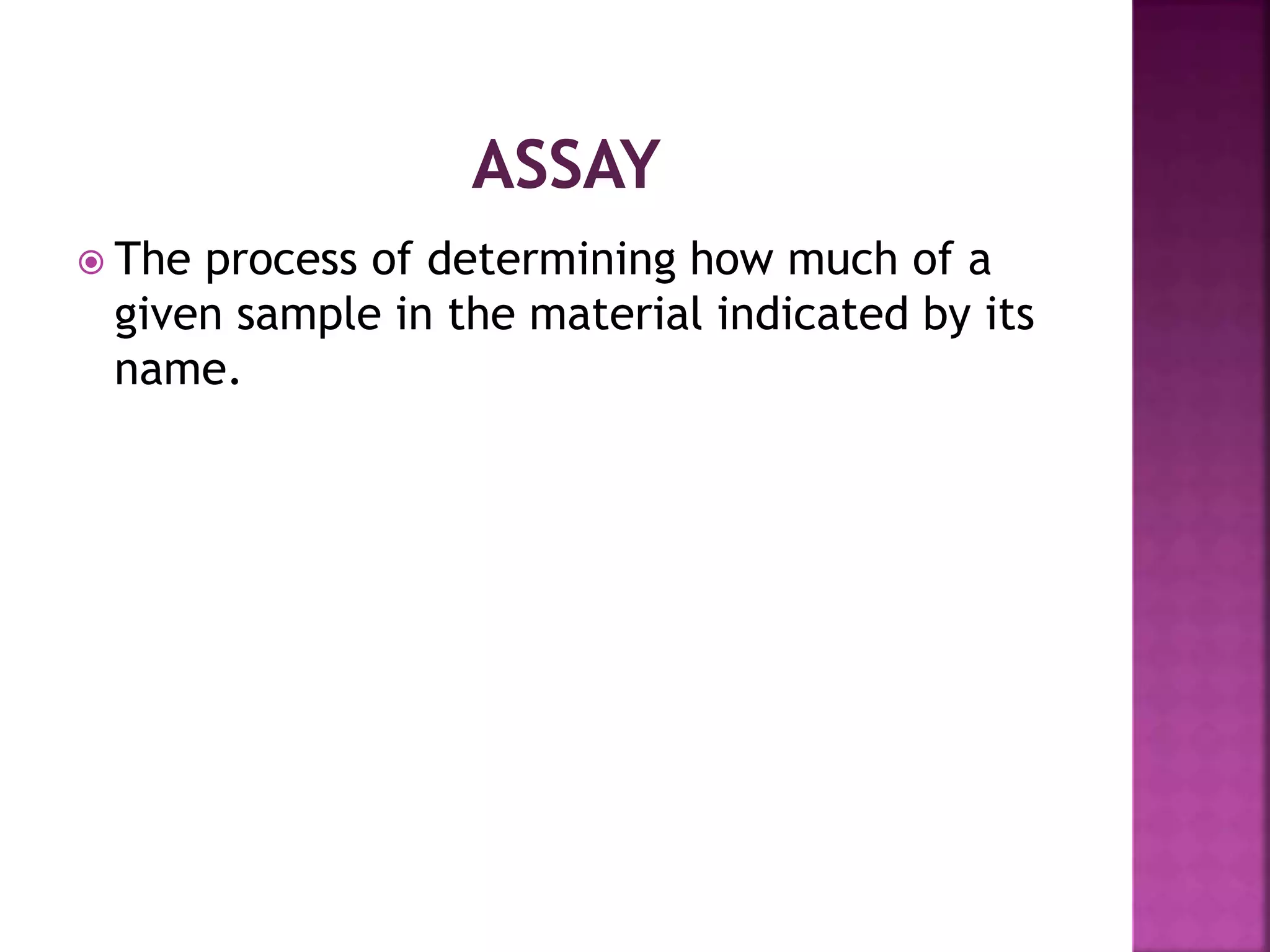
It is sub discipline of the analytical
chemistry covering the quantitative
measurement of xenobiotics [ drugs
,metabolites and biological molecule in the
unnatural location or concentration] and
biotics [macromolecules, proteins, DNA,
metabolites] in biological system](https://image.slidesharecdn.com/extractionofbioanalyticalsample-201121083657/75/Extraction-of-bioanalytical-sample-8-2048.jpg)