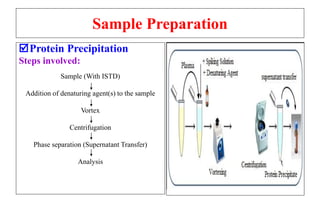
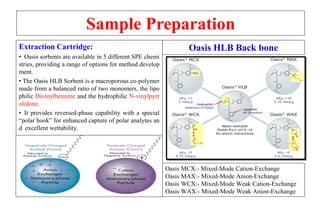
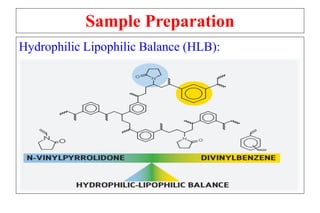
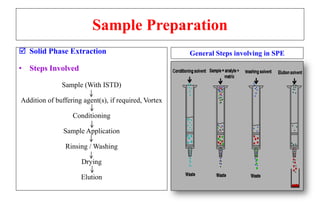
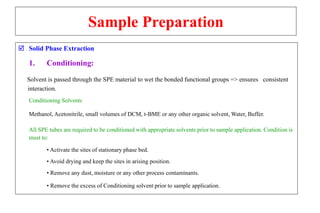
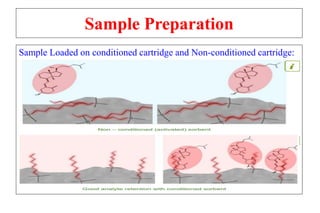
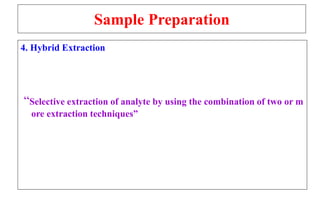
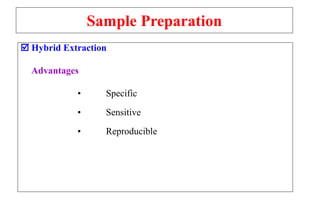
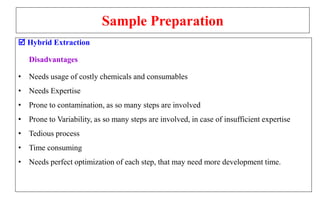
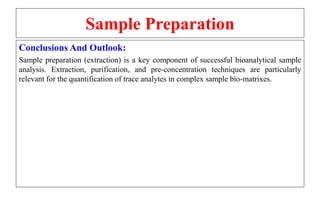
The document outlines the bioanalytical sample preparation process, detailing its importance in ensuring samples are clean, concentrated, and suitable for analysis. It describes various techniques such as protein precipitation, liquid-liquid extraction, solid-phase extraction, and hybrid extraction, highlighting their principles, advantages, and disadvantages. Additionally, it emphasizes the necessity of proper training and adherence to good laboratory practices during sample preparation to mitigate risks and improve outcomes.