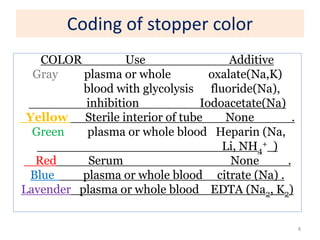
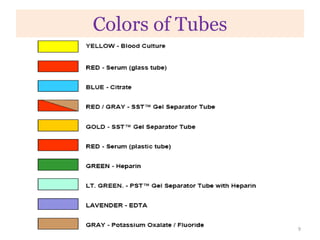
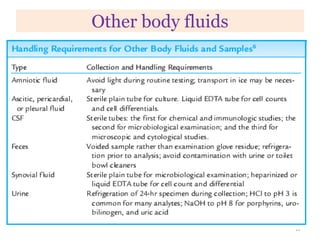
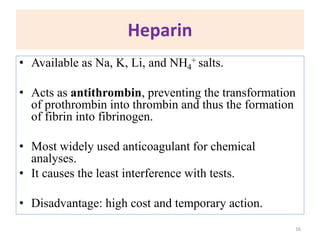
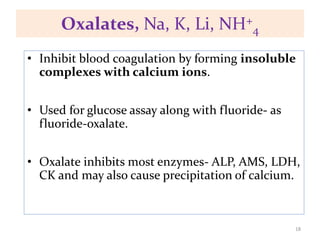
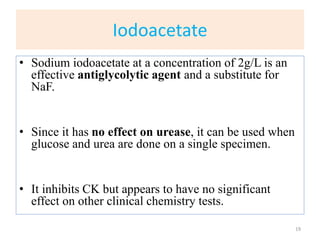
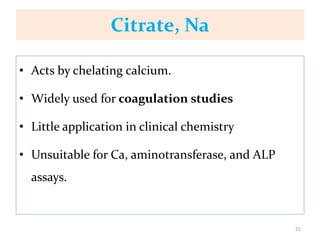
This chapter discusses specimen collection, processing, and preservation for biochemical analysis. It describes the importance of proper collection and labeling of specimens and lists common specimen types including blood, urine, and body fluids. The chapter outlines factors that affect blood composition and details methods for blood collection as well as the use of anticoagulants and tubes with different colored stoppers. It also discusses the preservation of specimens through refrigeration, freezing, and use of chemical preservatives to inhibit reactions prior to analysis. Finally, the chapter reviews preanalytical factors that can influence test results such as collection procedures, sample handling and processing, and the presence of hemolysis.