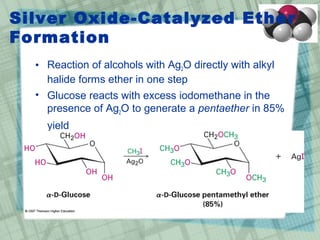
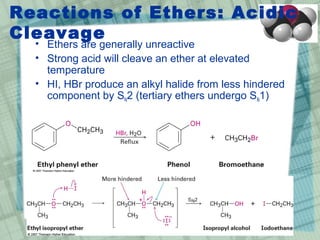
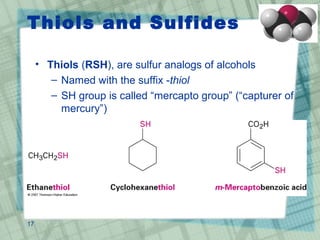
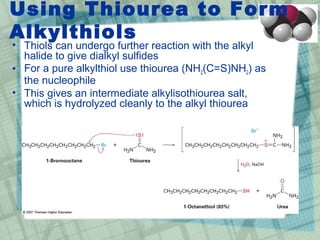
The document discusses ethers, epoxides, and sulfides, highlighting their structures, synthesis methods, and reactions. It explains how ethers have two organic groups attached to an oxygen atom and details various synthesis techniques like the Williamson ether synthesis and the formation of epoxides. Additionally, the document covers the properties and reactions of thiols and sulfides, emphasizing their nucleophilicity and oxidation processes.