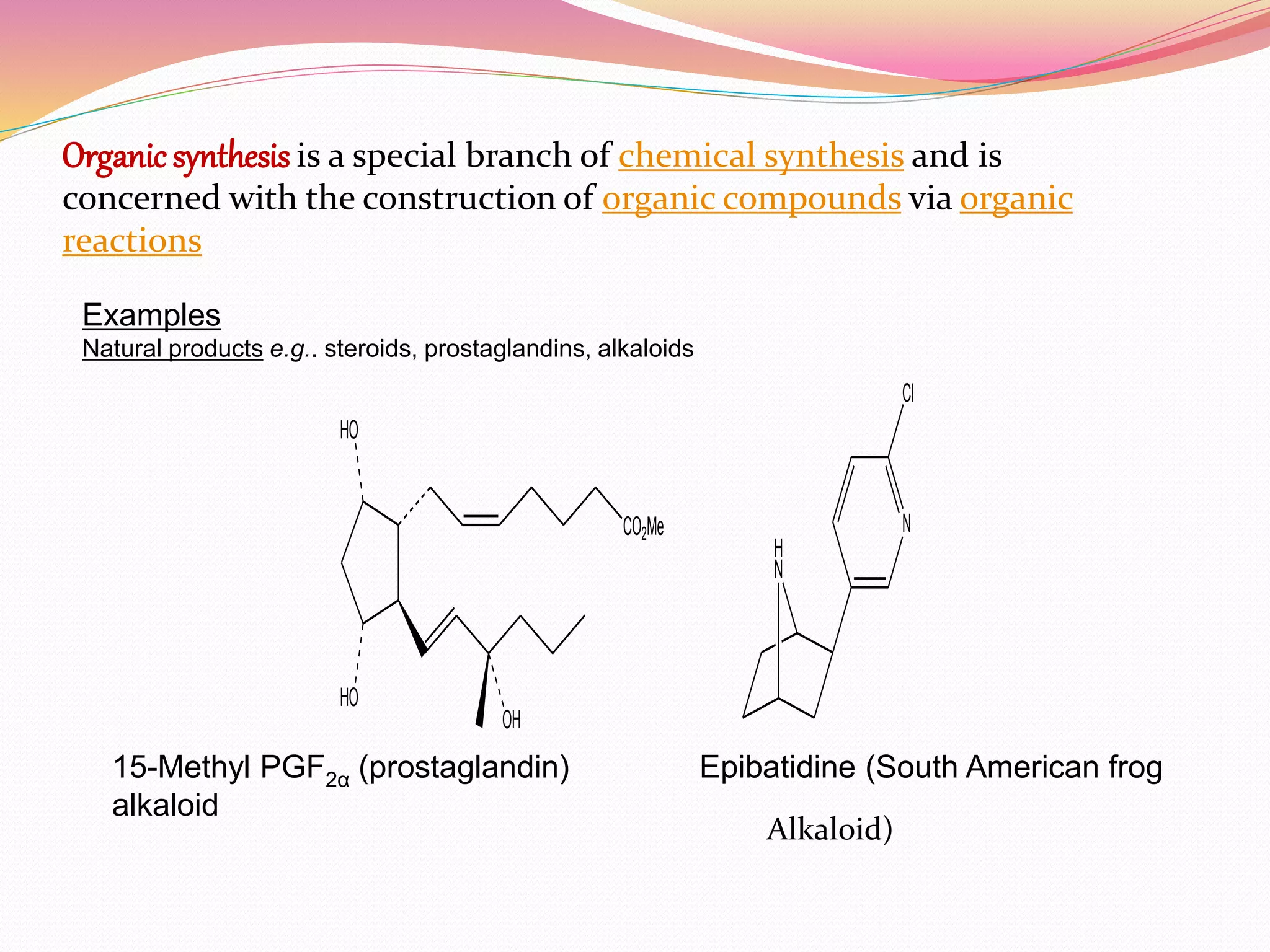
The document discusses methodology in organic synthesis, including examples of natural products. It describes convergent and divergent synthesis strategies. Convergent synthesis involves coupling molecular fragments through independent synthesis to improve reaction yields compared to linear synthesis. Divergent synthesis starts from a central core and generates a library of compounds through successive additions. Functional group interconversion and addition techniques are discussed to allow for disconnection of target molecules during retrosynthetic analysis.




![o An example of its use in total synthesis is the final step
(photochemical [2+2]cycloaddition) towards the compound
Biyouyanagin A:
o Proteins of up to 300 amino acids are produced by a
convergent approach using chemical ligation.
Examples:](https://image.slidesharecdn.com/methodologyoforganicsynthesis-1-160121042922/75/Methodology-of-organic-synthesis-6-2048.jpg)












