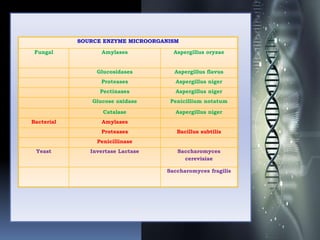
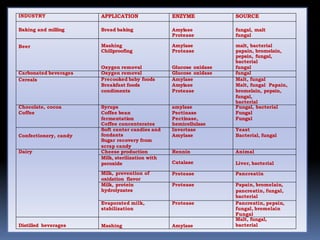
The document discusses microbial enzymes. It begins by defining enzymes as biological catalysts produced by living organisms. It then discusses the history of enzymes, noting that Wilhelm Kühne first coined the term in 1877. The document outlines various sources of microbial enzymes from soil, food, and degrading areas. Common microorganisms that produce enzymes include bacteria, fungi, and actinomycetes. Examples are provided of specific enzymes produced by different microorganisms. The document then discusses methods for isolating and purifying enzymes, including precipitation, chromatography techniques, and affinity purification. It provides examples of enzyme purification strategies and industrial applications of enzymes in areas like baking, brewing, dairy, pharmaceuticals, and more.