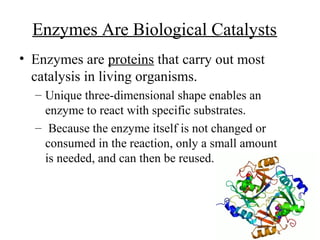
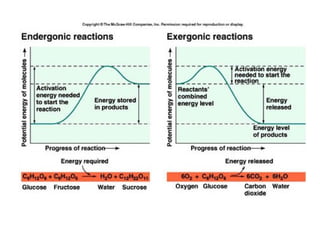
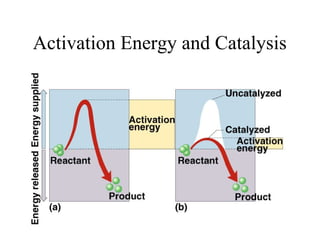
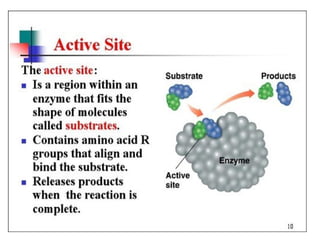
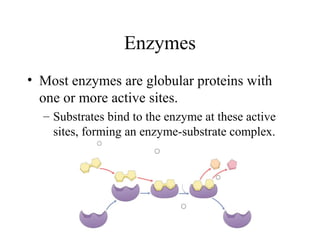
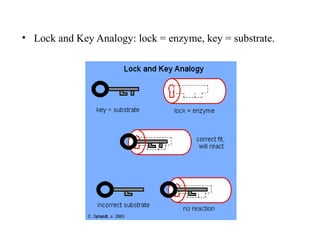
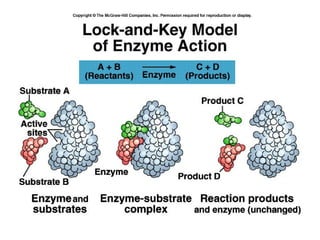
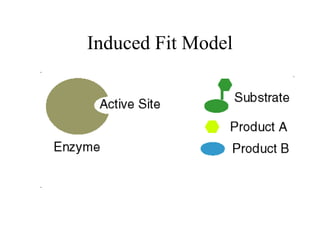
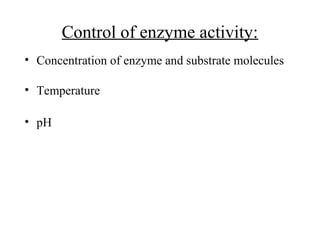
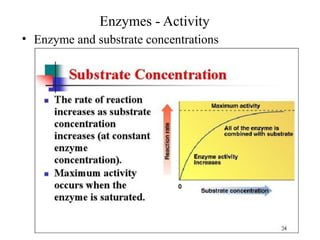
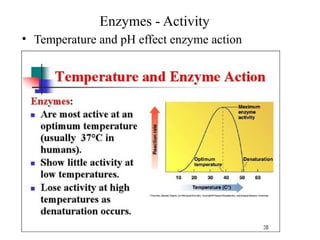
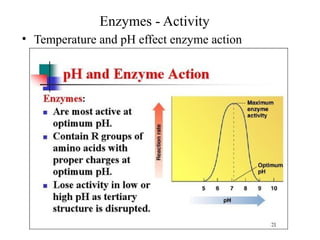
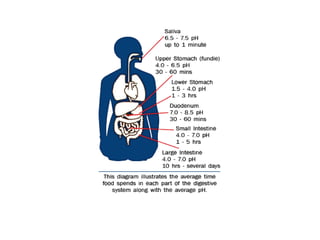
Enzymes are proteins that act as biological catalysts and are necessary for most chemical reactions in living cells. They help reactions occur by lowering their activation energy. Enzymes have a unique three-dimensional shape with active sites that allow them to specifically bind to substrates and rearrange the molecules involved in chemical steps. While they are not consumed in these reactions, enzymes increase the rates of reactions and can be reused, making them highly efficient biological catalysts.