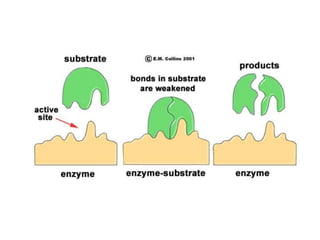
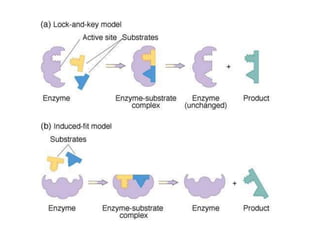
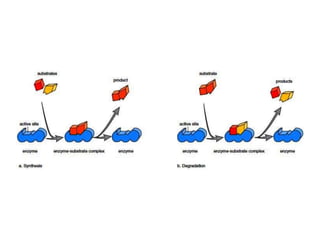
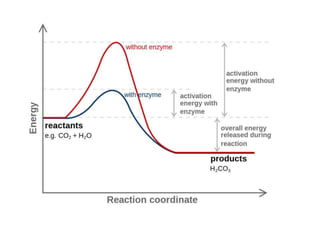
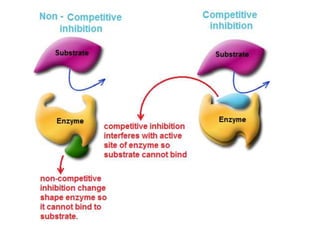
Enzymes are globular proteins that catalyze metabolic reactions. They have an active site that binds to substrate molecules in a "lock and key" fashion. This lowers the activation energy needed for reactions to occur. Enzyme activity is affected by concentration, temperature, pH, and inhibitors. Inhibitors bind to the active site and prevent substrate binding, competitively or non-competitively. This inhibition allows control of reaction pathways and is important in processes like glycolysis.