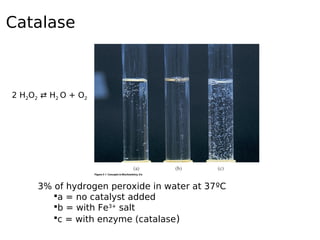
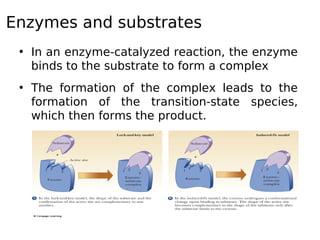
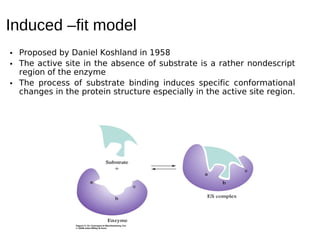
This document discusses enzymes and their role in catalysis. It defines enzymes as biological catalysts that speed up metabolic reactions in the body. Enzymes work by lowering the activation energy of reactions, allowing reactants to more easily form transition states and products. The document explores early models of enzyme-substrate binding, including the lock and key model and induced fit model. It also discusses concepts like activation energy profiles and how enzyme assays are used to measure enzyme activity.