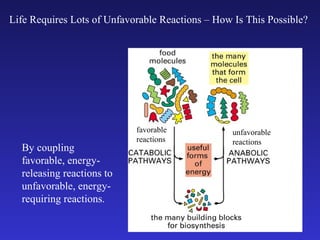
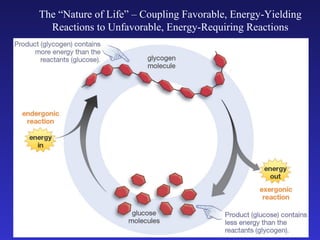
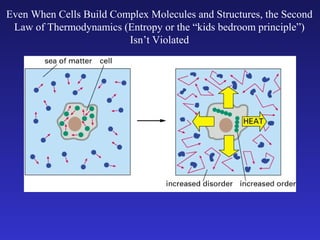
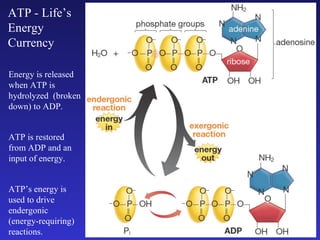
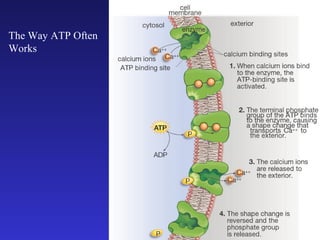
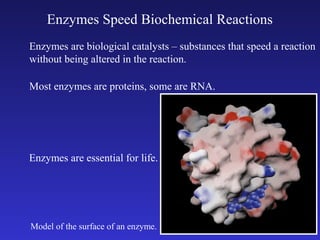
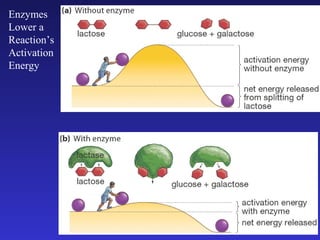
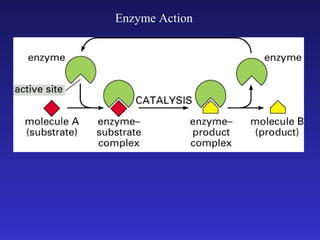
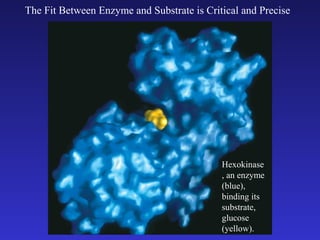
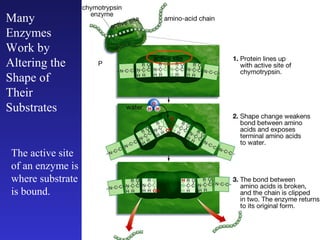
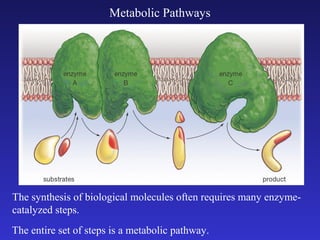
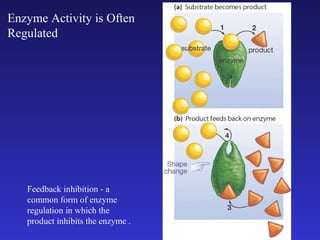
The document discusses how life requires energy derived primarily from sunlight via photosynthesis. It then explains that life can be viewed as coordinated chemical reactions, and that enzymes play a key role in allowing these reactions to occur by lowering their activation energy. Specifically, enzymes allow organisms to couple favorable, energy-releasing reactions to unfavorable, energy-requiring reactions through the use of ATP as an energy currency.