This document discusses biochemical reactions and enzyme structure and function. It provides the following key points:
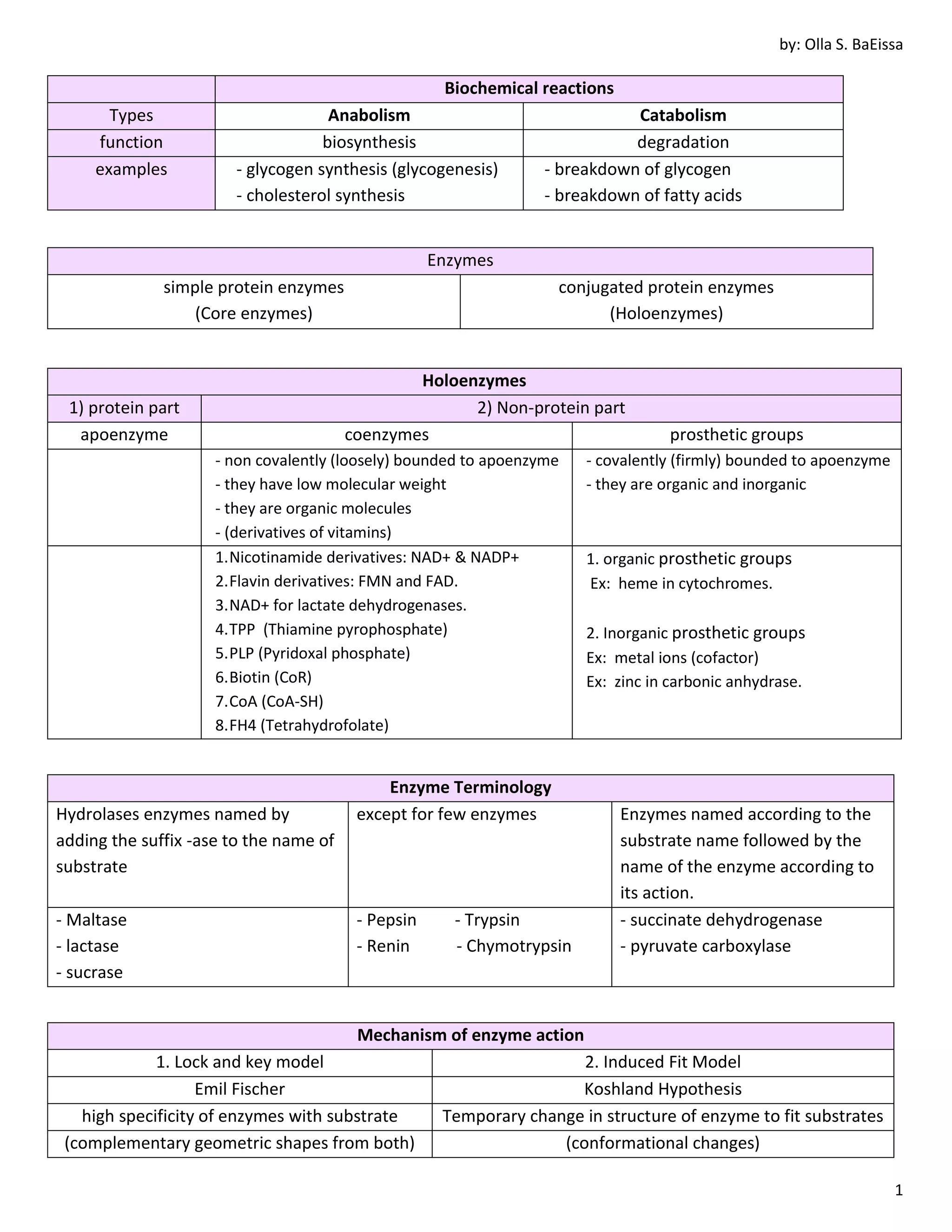
1. Biochemical reactions are divided into anabolism and catabolism. Anabolism involves biosynthesis while catabolism involves degradation. Enzymes are protein catalysts that facilitate biochemical reactions.
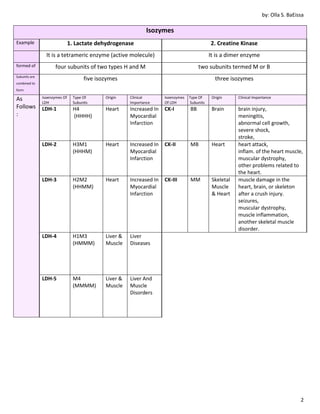
2. Enzymes can be simple protein enzymes or conjugated protein enzymes called holoenzymes that contain a protein part and a non-protein part like coenzymes or prosthetic groups. Isozymes are enzymes that catalyze the same reaction but have different structures and properties.
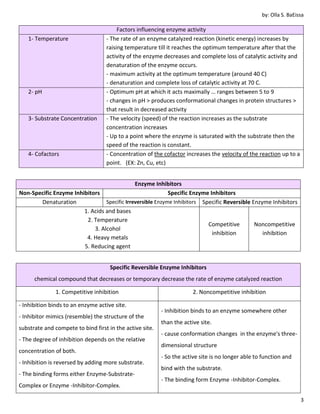
3. Factors like temperature, pH, substrate concentration, and cofactors can influence enzyme activity by altering