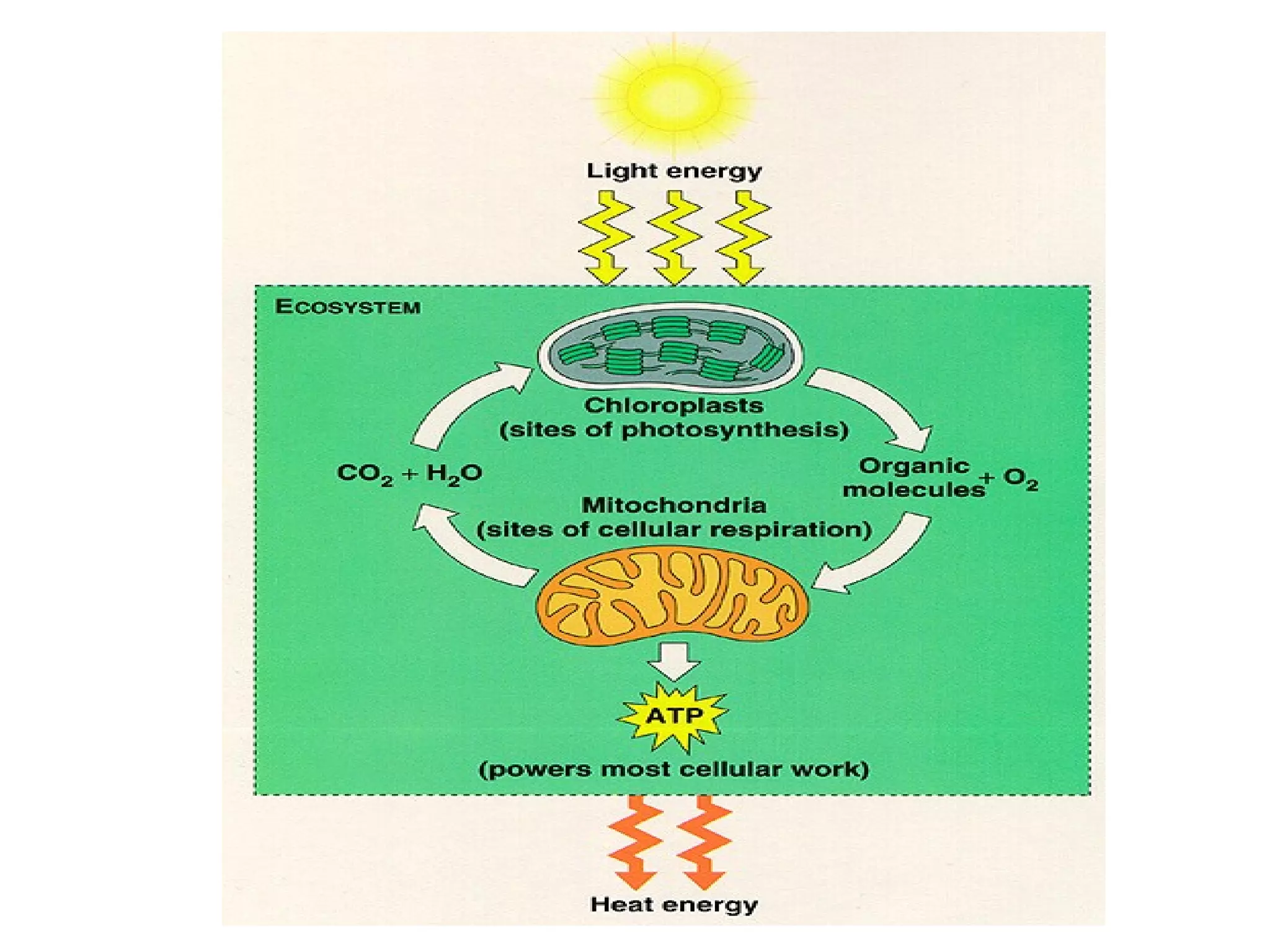
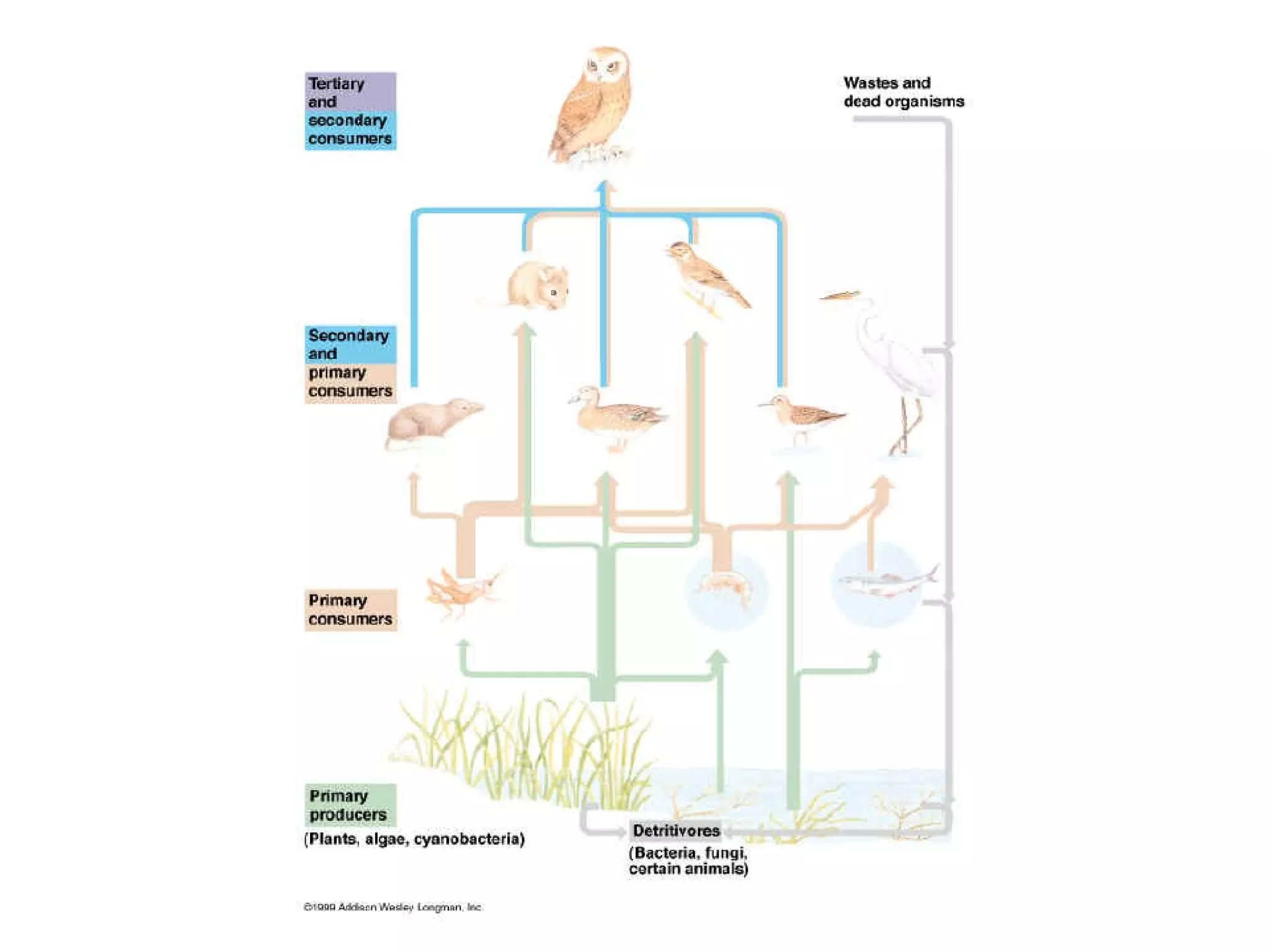
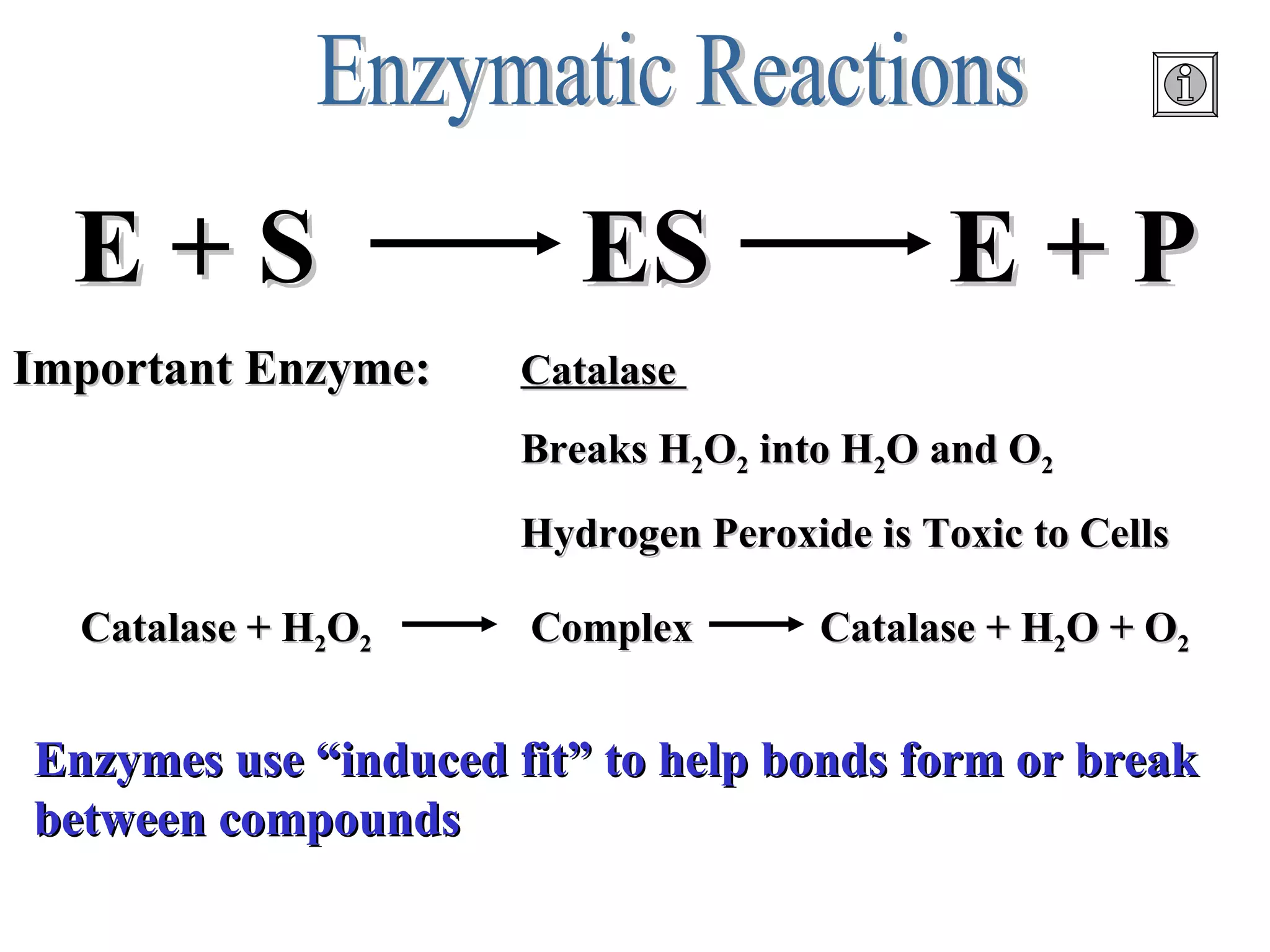
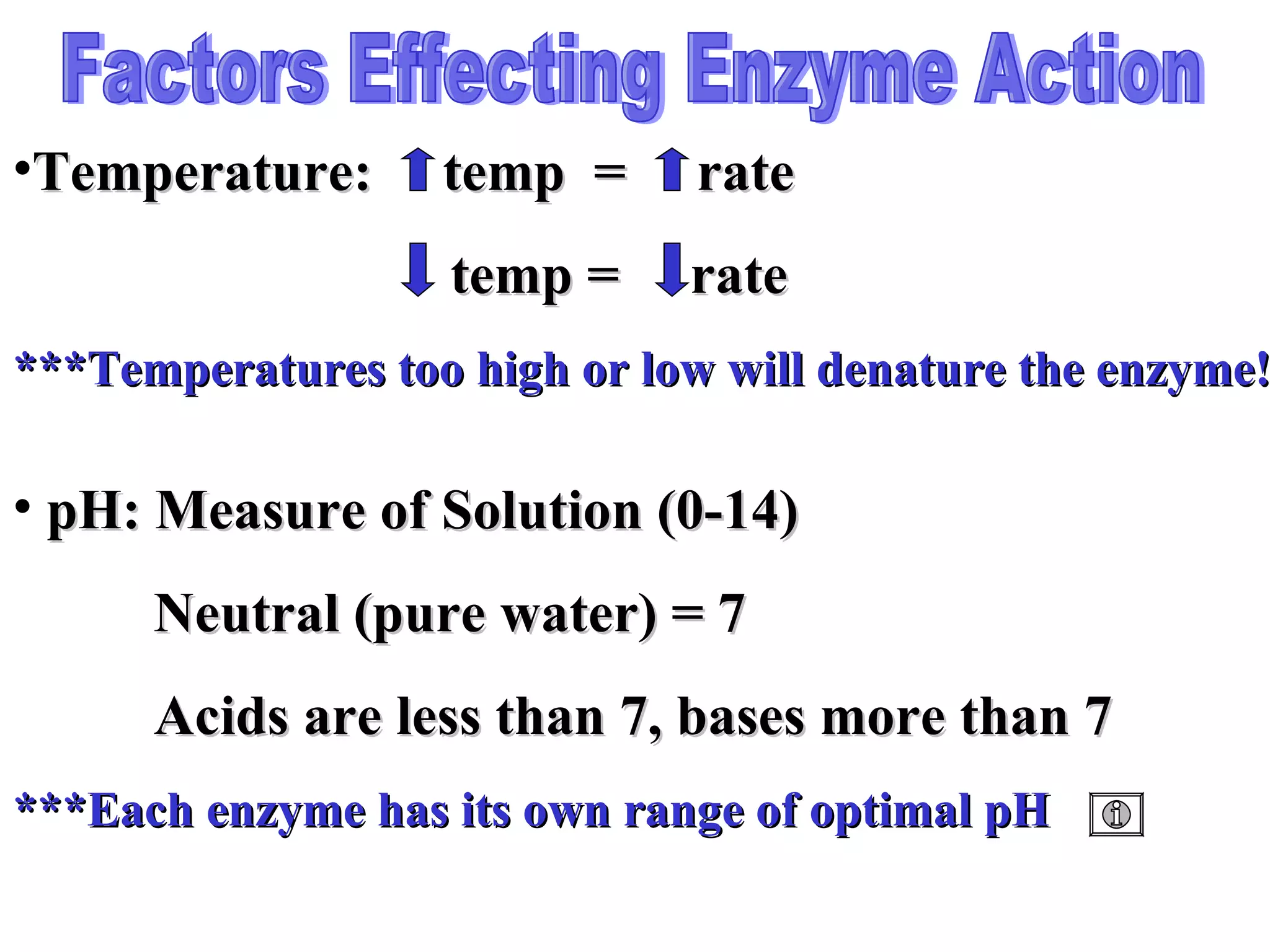
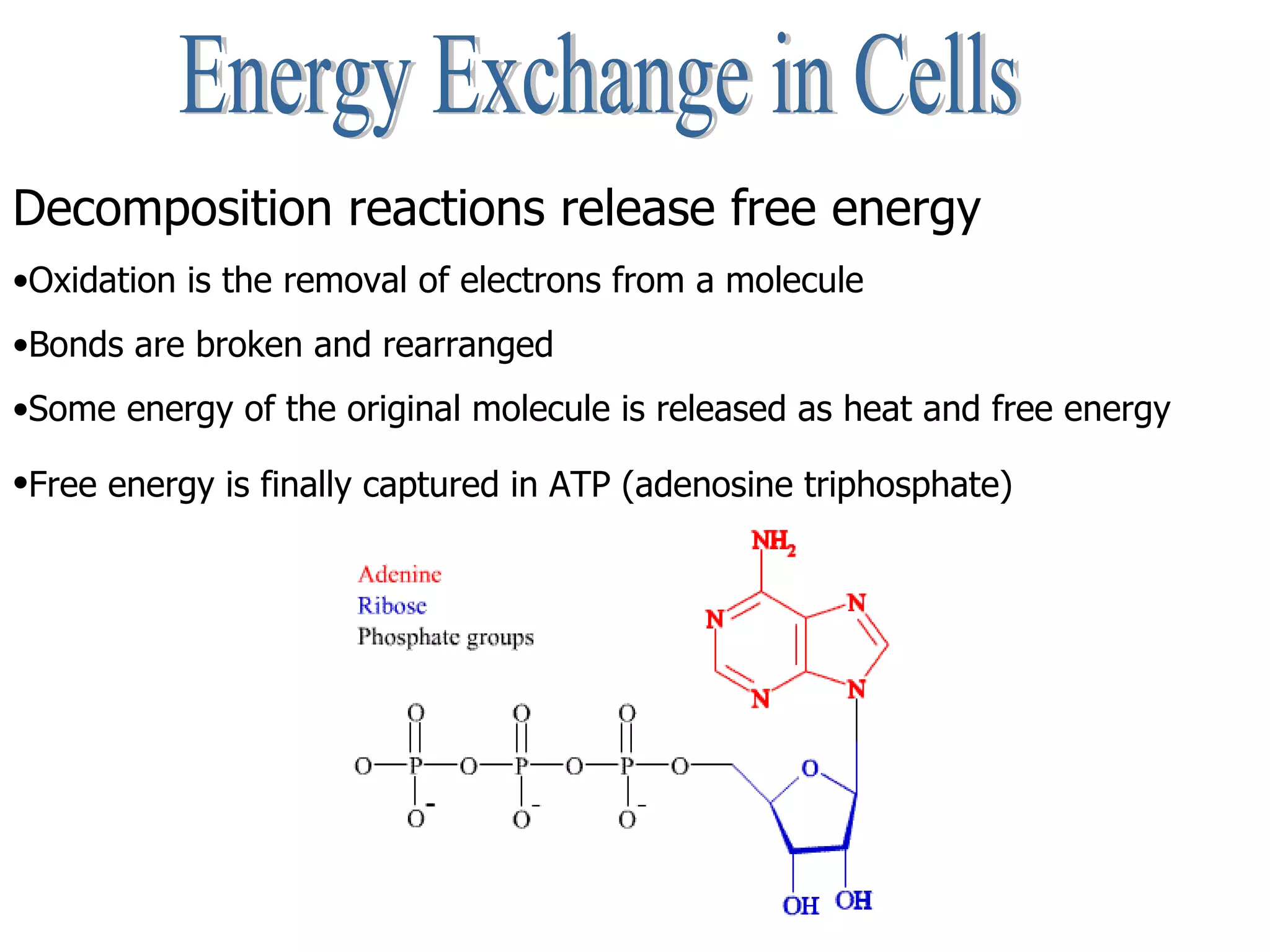
The document discusses the role of energy in organisms, emphasizing that energy is essential for chemical reactions and must be obtained from external sources. It highlights the importance of enzymes as catalysts that speed up reactions by lowering activation energy, and explains metabolic processes such as synthesis and decomposition. Additionally, it covers factors affecting enzyme activity, including pH and temperature, while explaining energy exchange in cellular processes like respiration and ATP production.