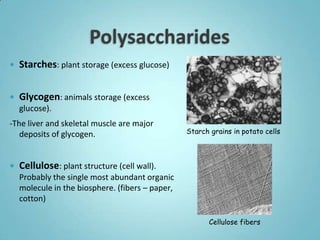
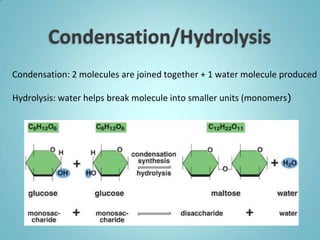
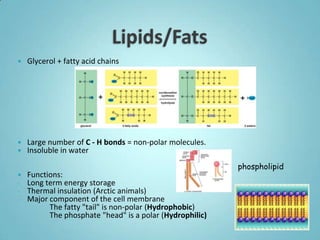
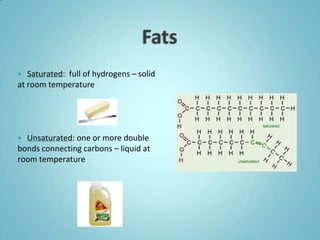
1. Organic molecules contain carbon and hydrogen. Water is the most abundant organic compound and is essential for life.
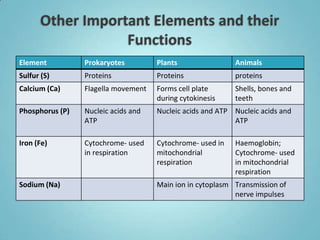
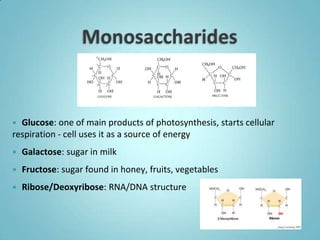
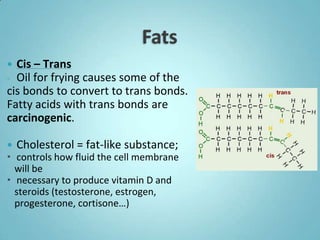
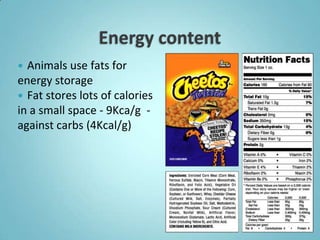
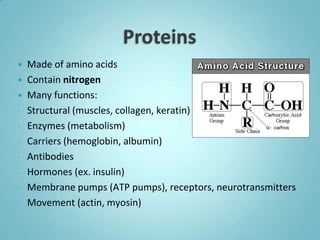
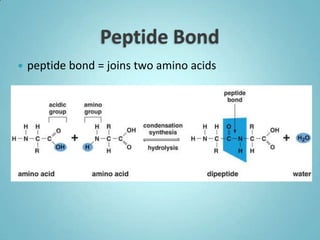
2. Sugars, lipids, proteins, and nucleic acids are the main types of organic molecules. Sugars provide short-term energy, lipids provide long-term energy storage, and proteins and nucleic acids have structural and informational roles.
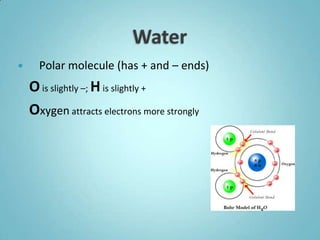
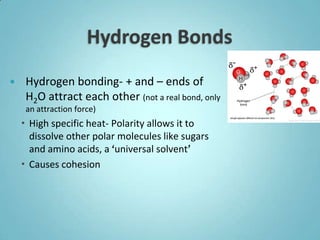
3. The properties of water, including its polarity, high heat capacity, and ability to dissolve many substances, make it uniquely suited to support life. Its properties allow it to regulate temperature, transport nutrients, and serve as a reaction medium for metabolic processes in organisms.