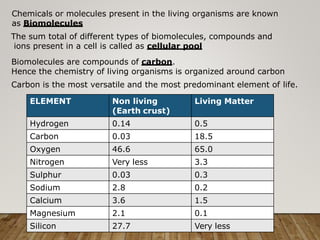
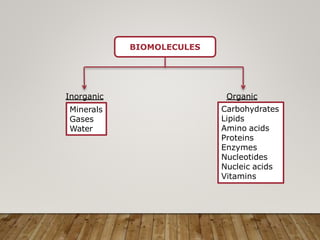
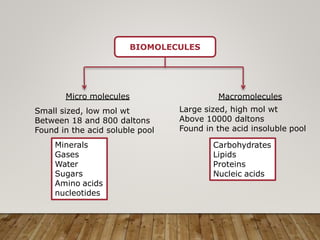
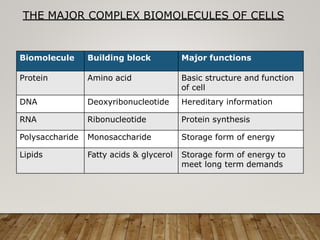
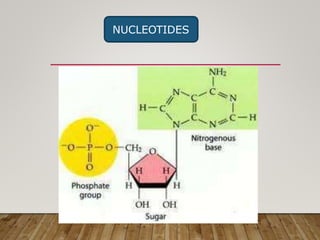
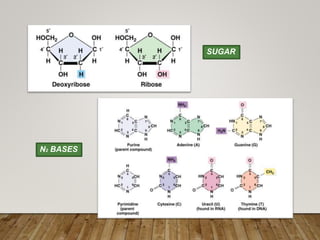
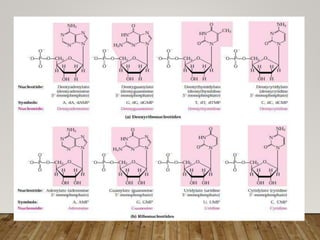
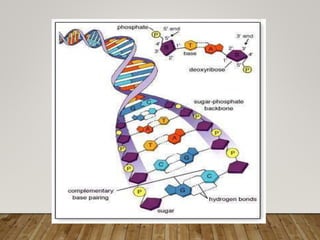
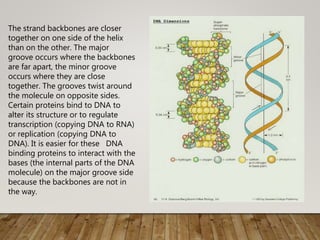
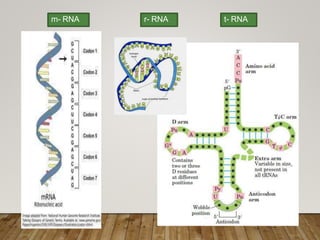
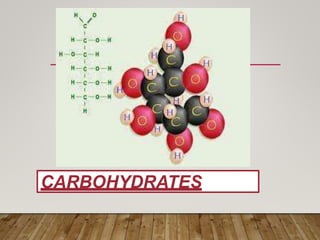
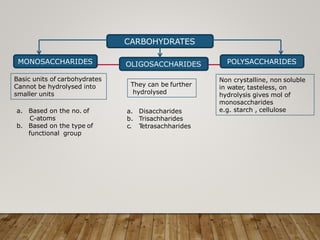
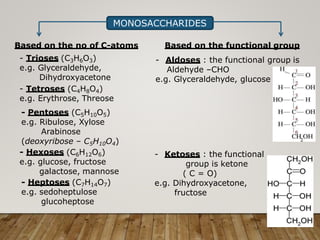
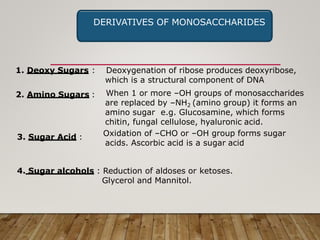
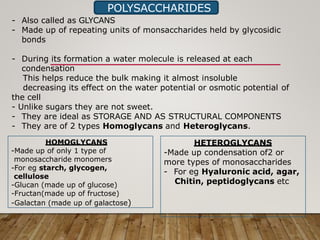
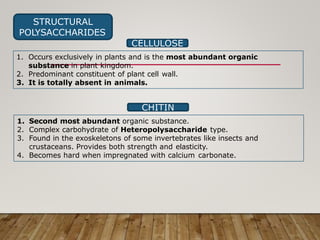
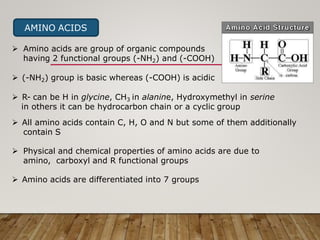
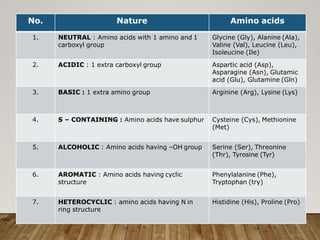
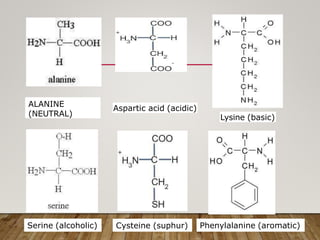
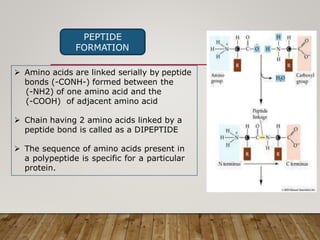
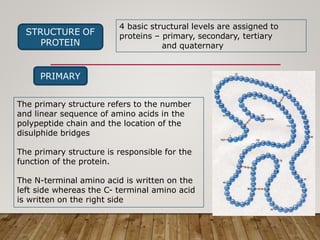
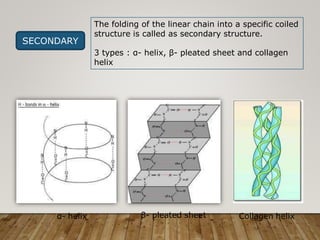
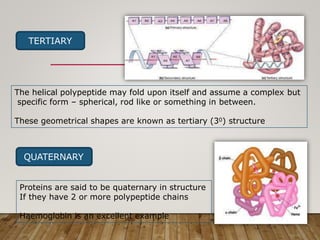
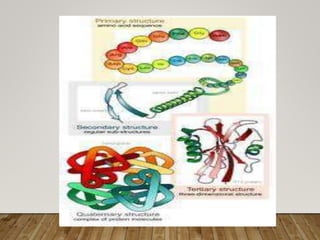
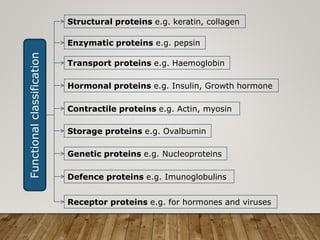
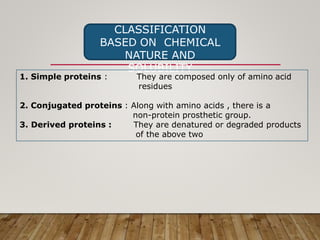
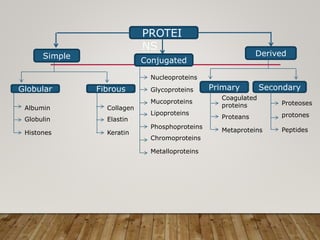
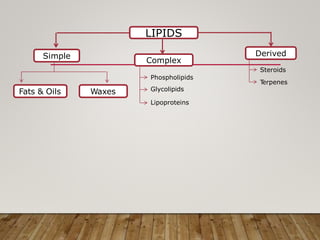
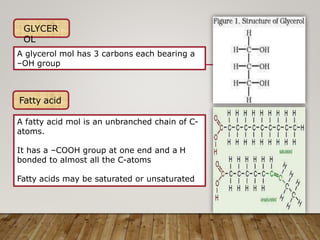
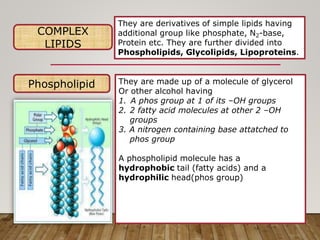
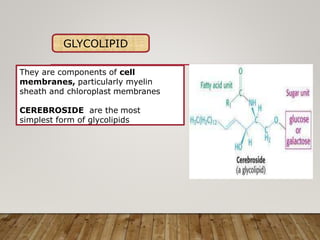
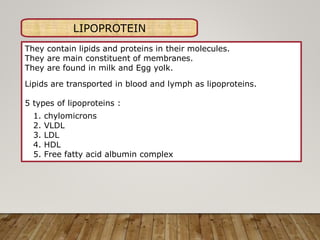
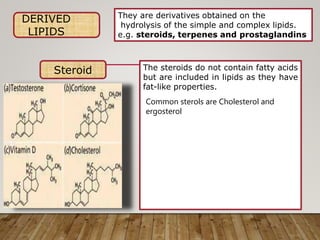
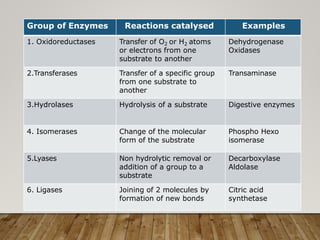
This document discusses biomolecules. It begins by defining biomolecules as chemicals or molecules present in living organisms. The sum total of different types of biomolecules, compounds, and ions present in a cell is called the cellular pool. Biomolecules are compounds of carbon, so the chemistry of living organisms is organized around carbon. The major biomolecules in cells include inorganic molecules like minerals and gases, organic molecules like carbohydrates, lipids, proteins, and nucleic acids, and smaller molecules like amino acids and nucleotides.