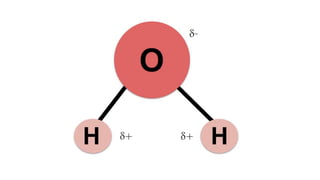
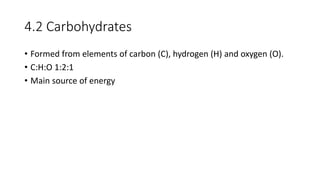
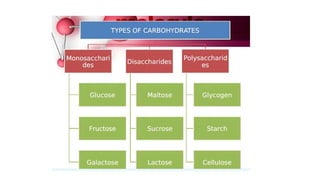
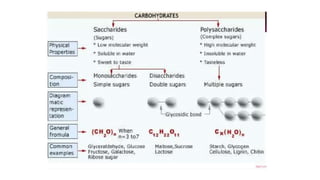
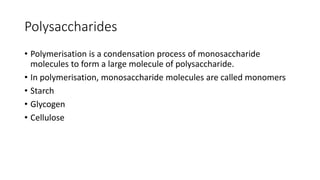
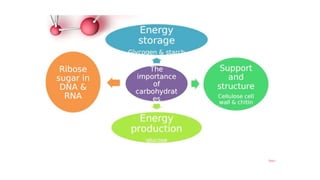
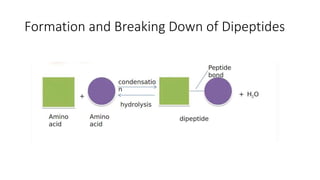
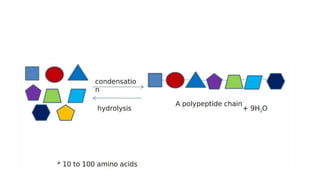
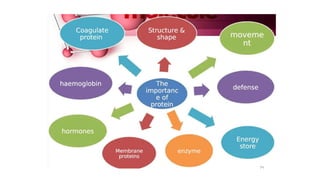
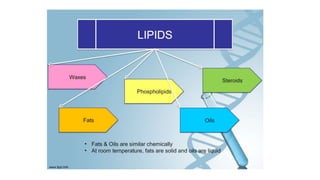
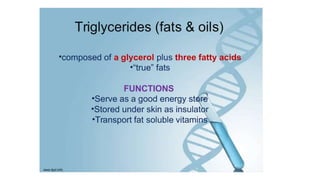
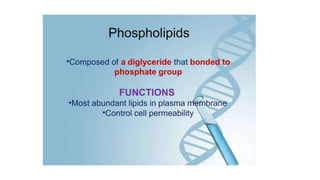
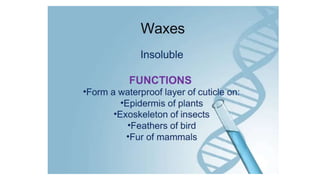
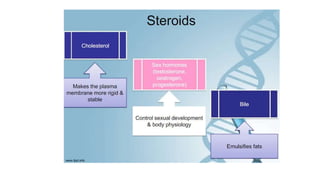
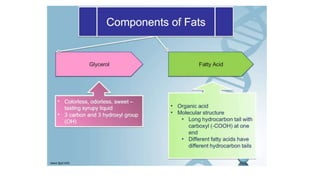
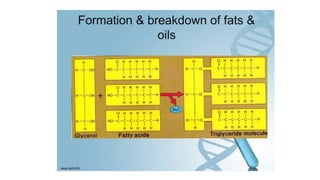
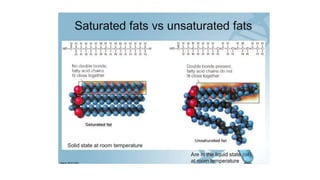
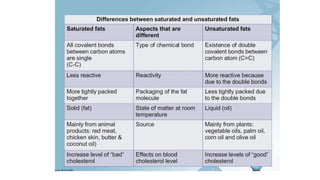
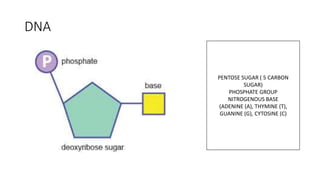
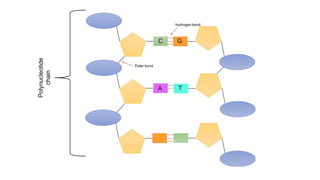
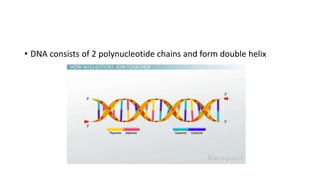
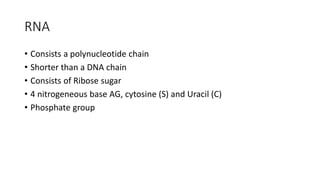
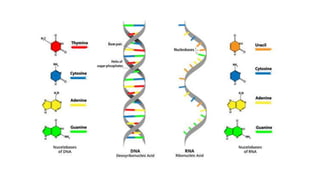
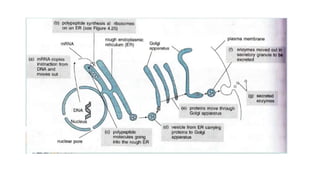
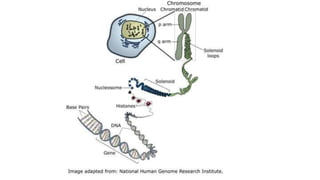
This document summarizes the key components of a cell's chemical composition. It discusses the main macromolecules that make up cells, including water, carbohydrates, proteins, lipids, and nucleic acids. Water is the most abundant component, making up 65-95% of plant cells and 80% of human cells. It plays important roles in temperature regulation, acting as a solvent, and transporting minerals within cells. Carbohydrates, proteins, and lipids are composed of carbon, hydrogen, oxygen, and other elements. They serve important functions like energy storage and insulation. Nucleic acids DNA and RNA contain genetic information and are involved in protein synthesis. DNA resides in the cell nucleus while RNA is found in the