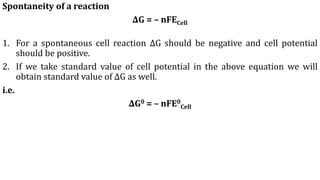
Electrochemistry is the study of chemical reactions that produce electricity and electrical energy's ability to cause non-spontaneous reactions. There are two types of electrochemical cells: galvanic cells that convert chemical energy to electrical energy, and electrolytic cells that use electrical energy to drive non-spontaneous reactions. Galvanic cells contain a spontaneous redox reaction like in Daniel cells where zinc oxidizes and copper reduces. Electrolytic cells use an external voltage to force nonspontaneous redox reactions. Standard electrode potentials allow prediction of reaction spontaneity based on the cell potential relative to the standard hydrogen electrode.