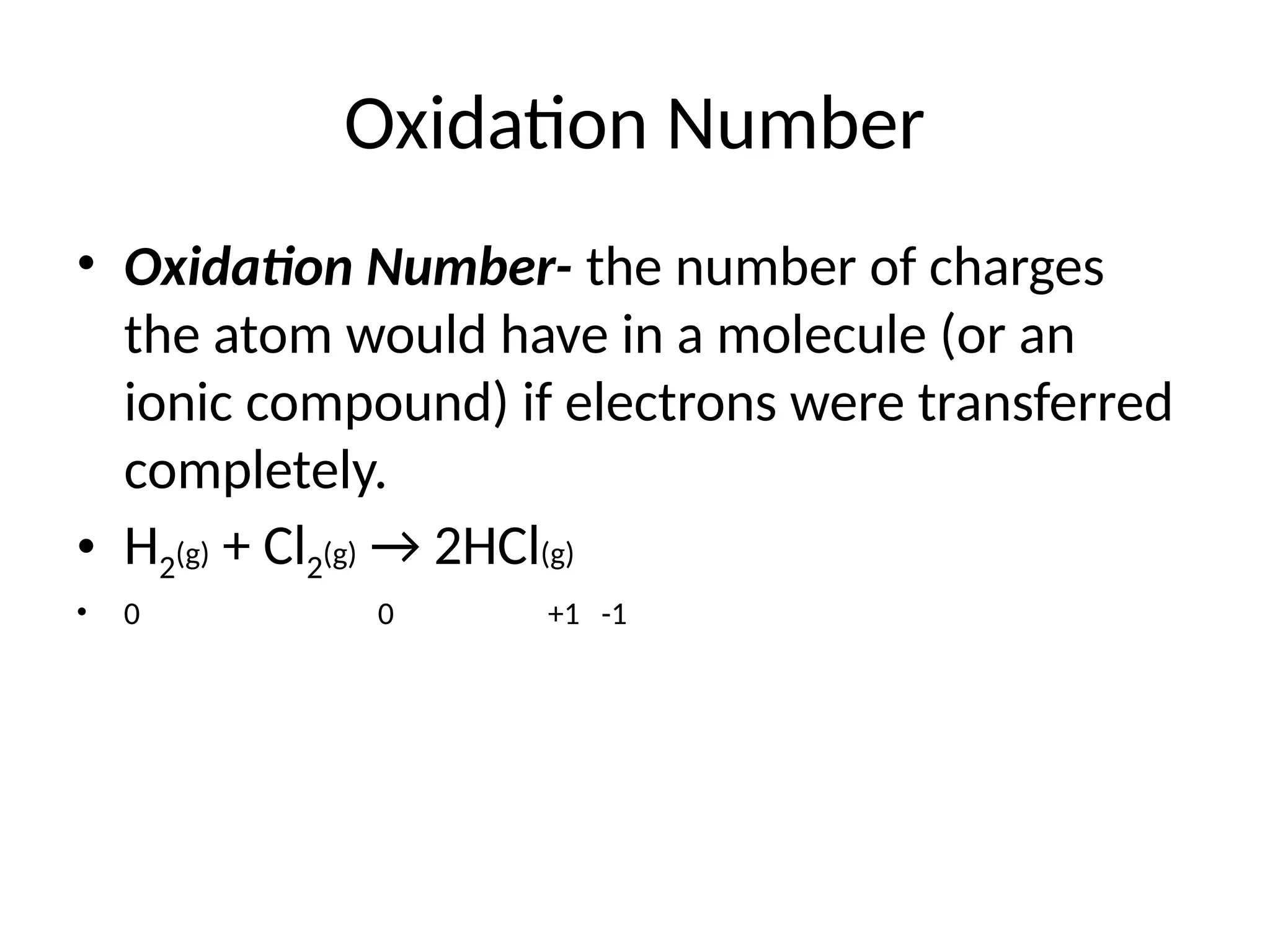
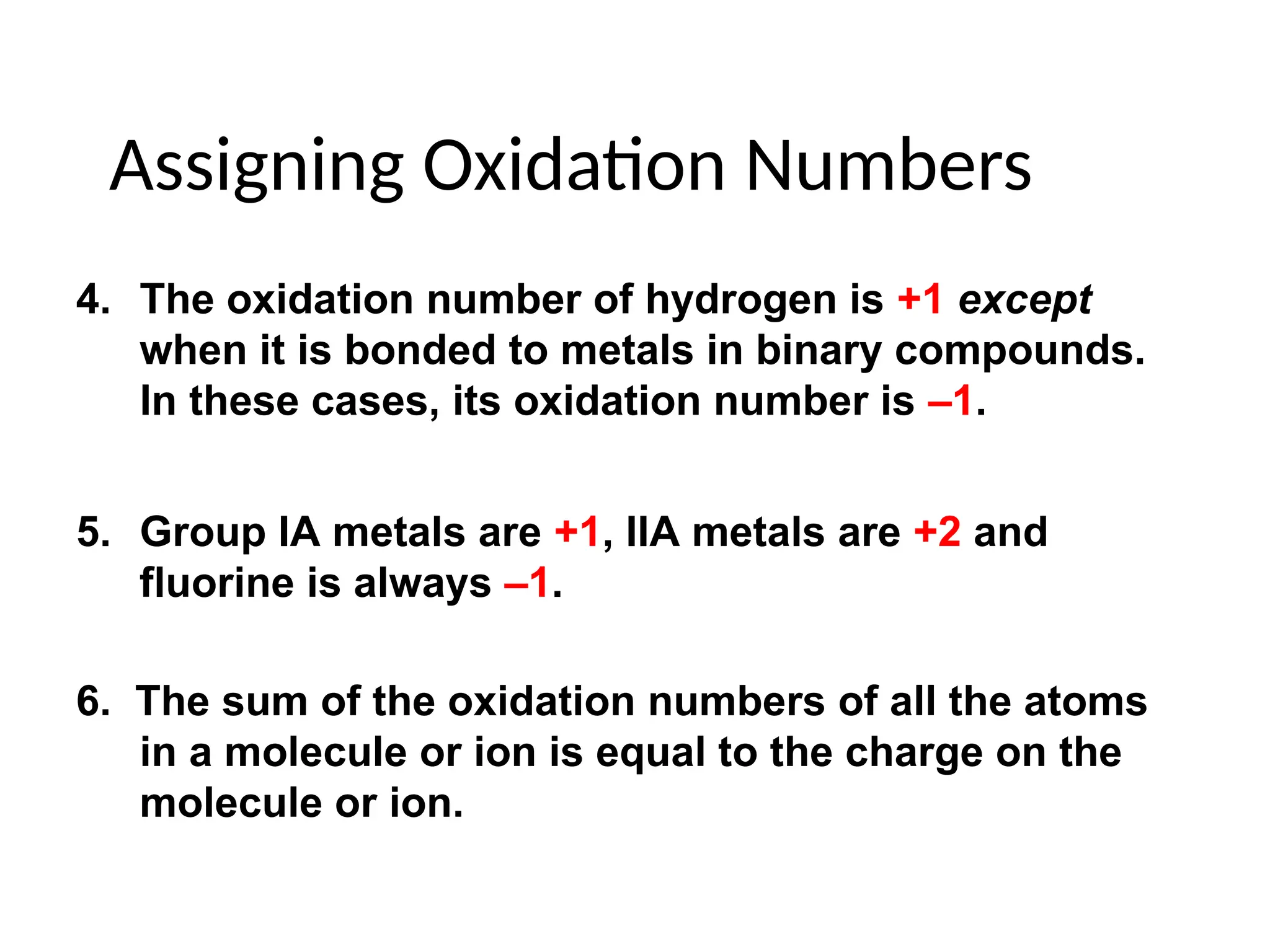
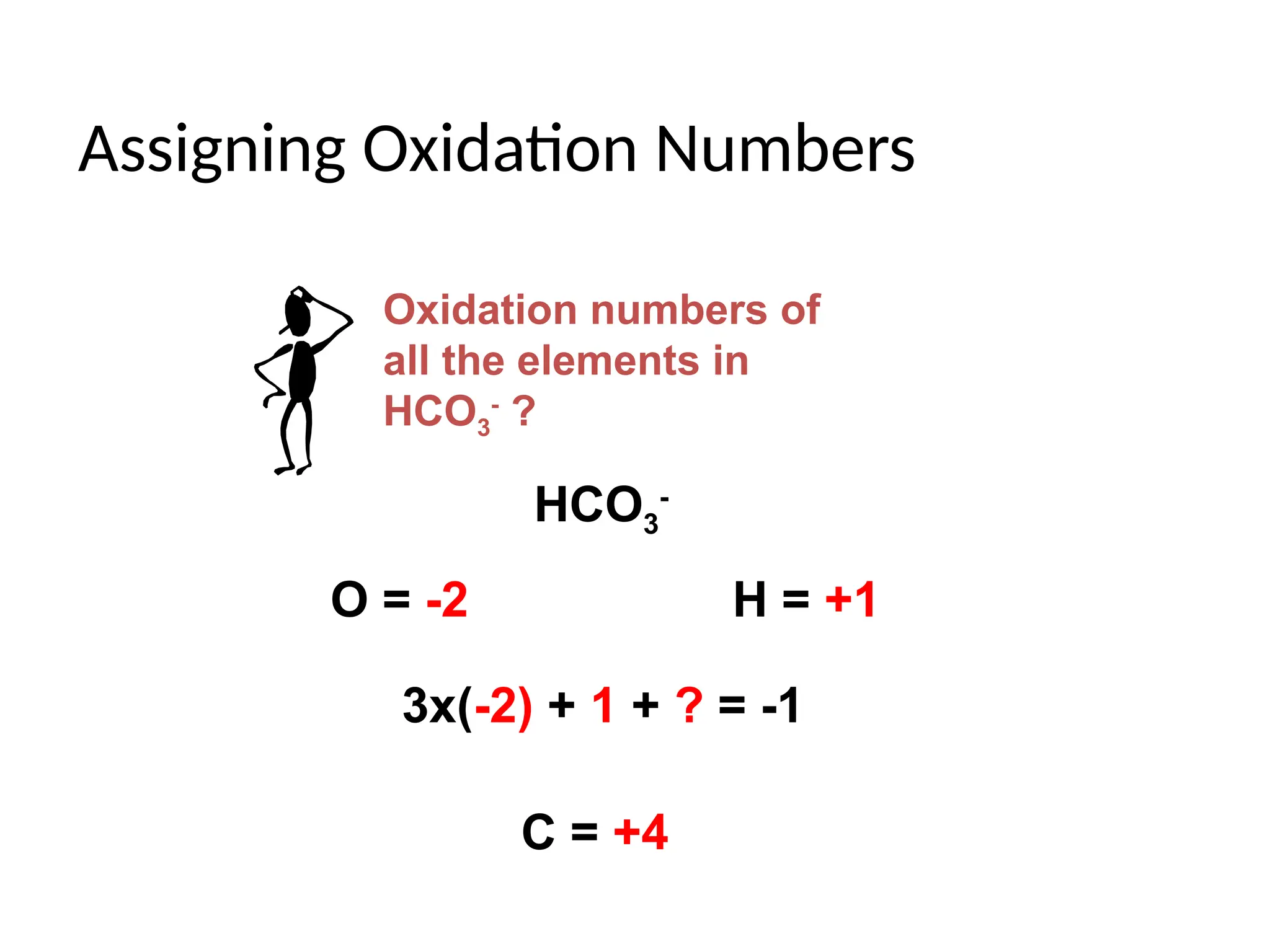
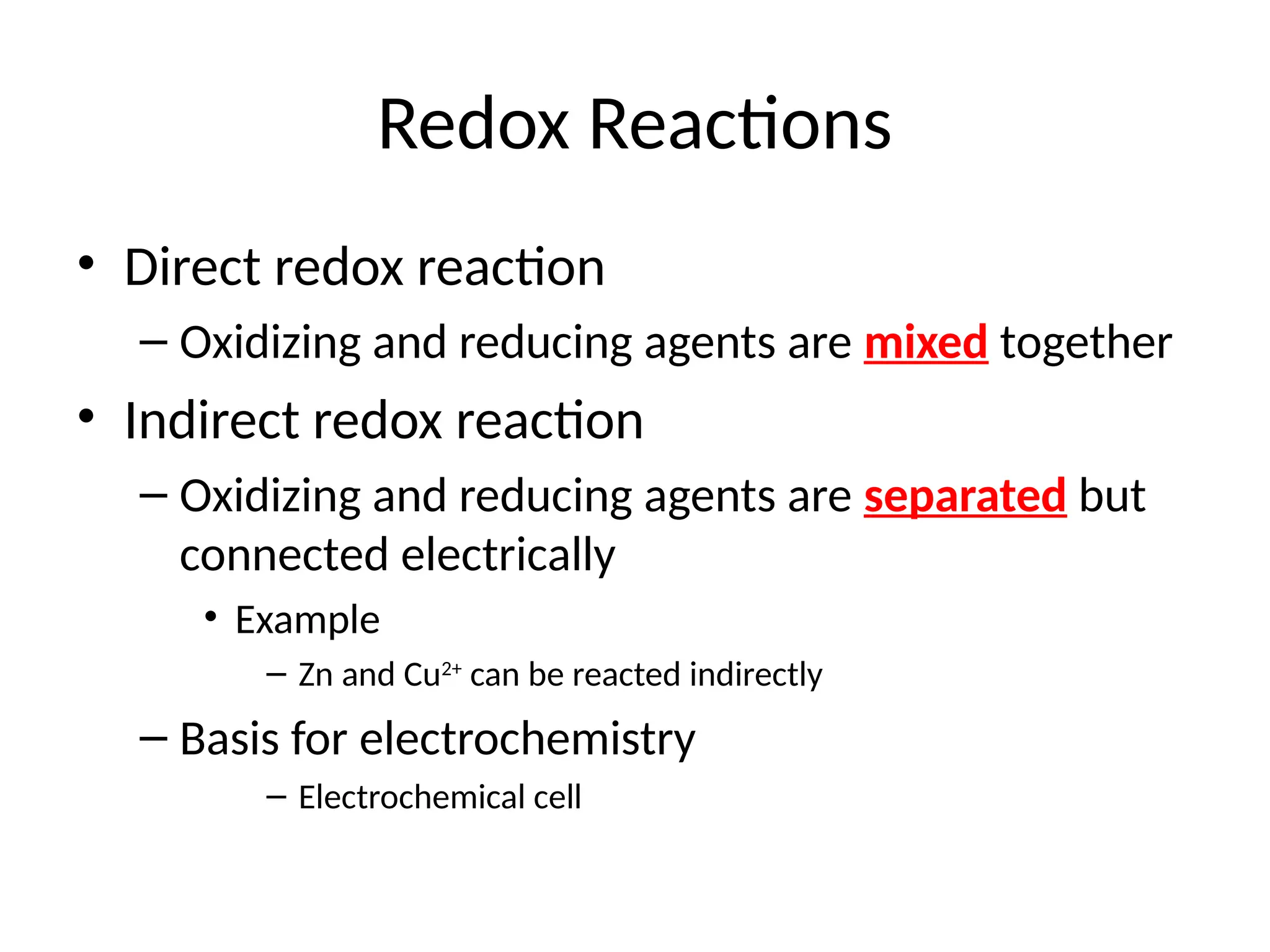
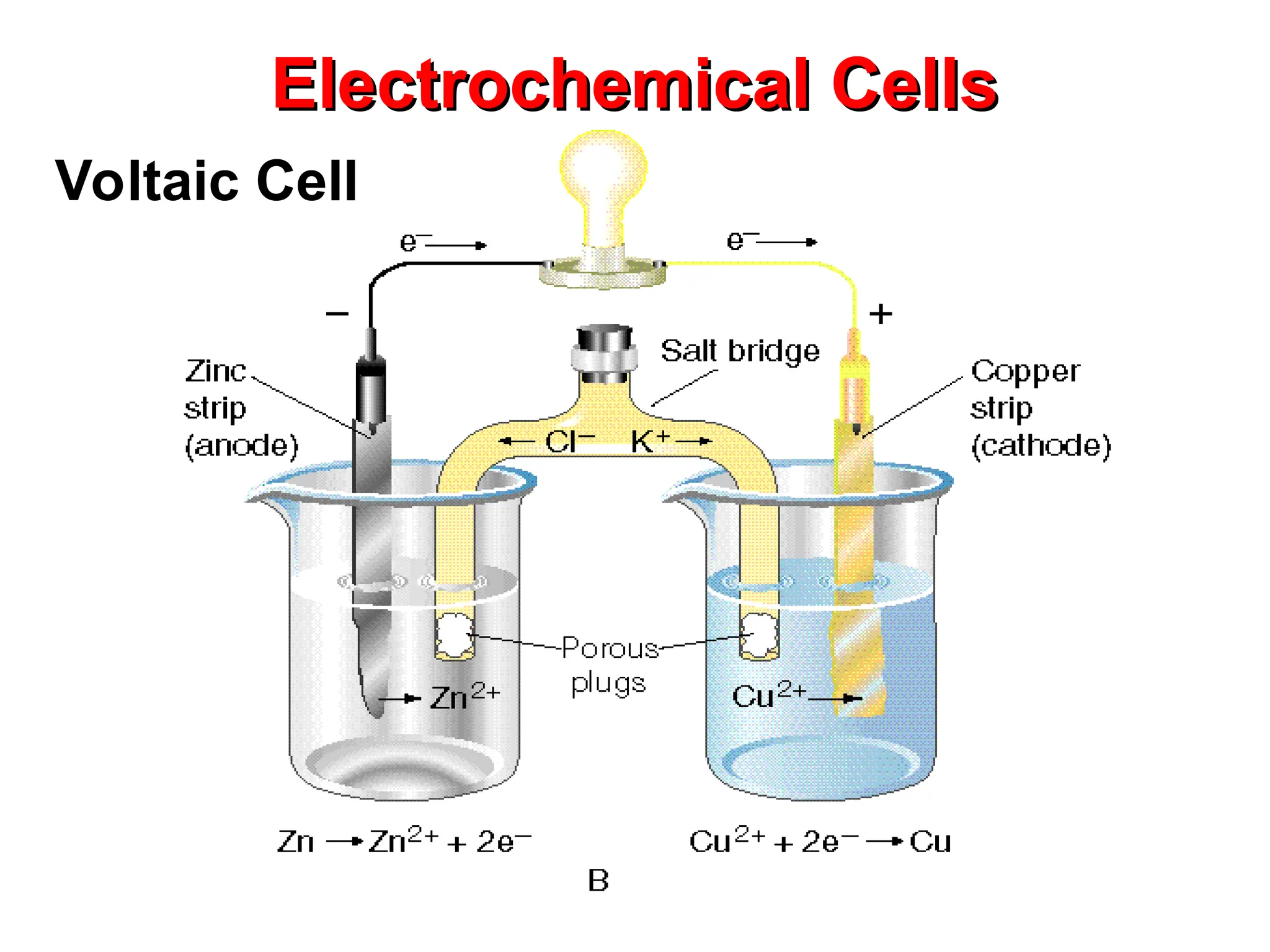
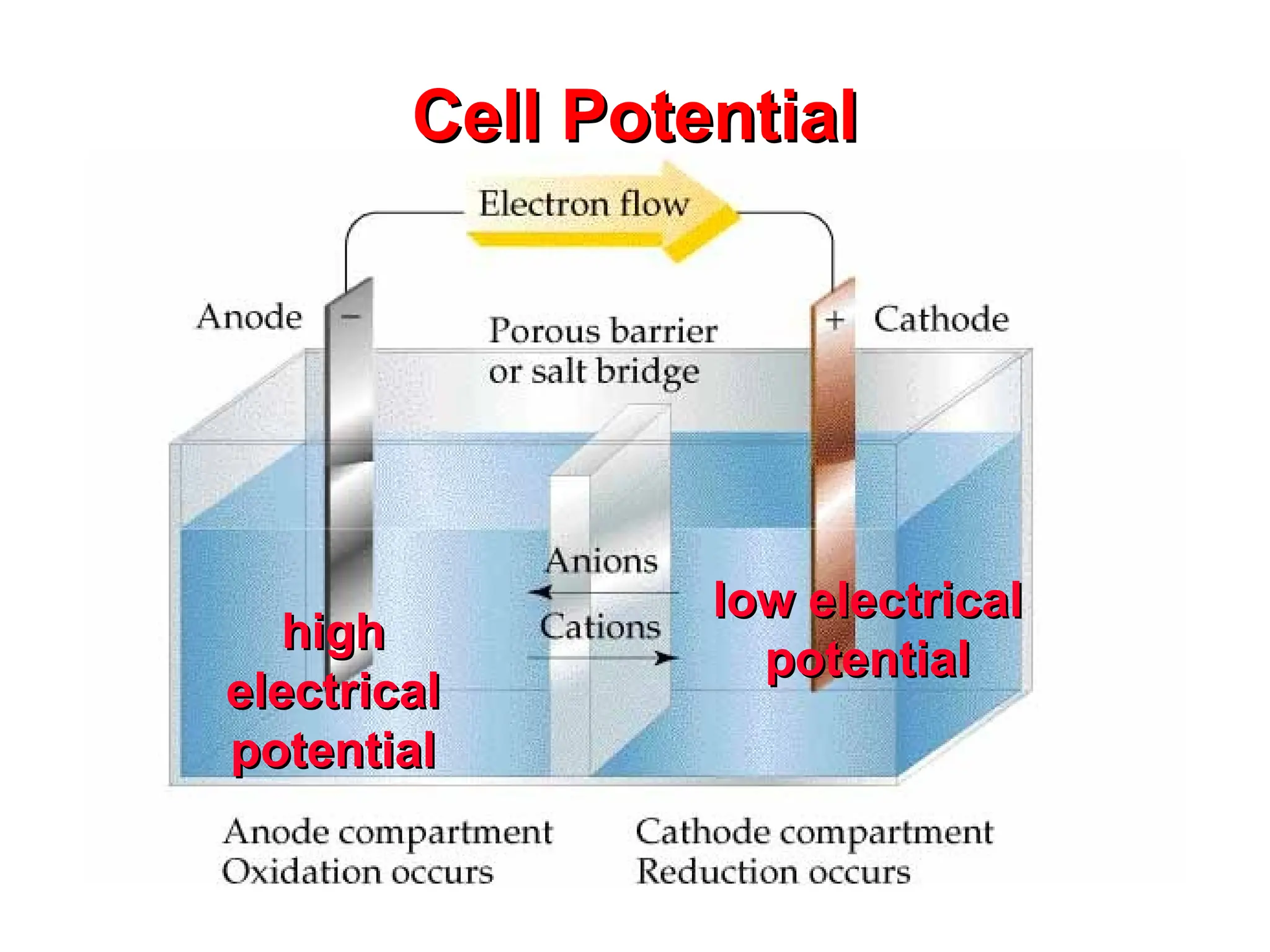
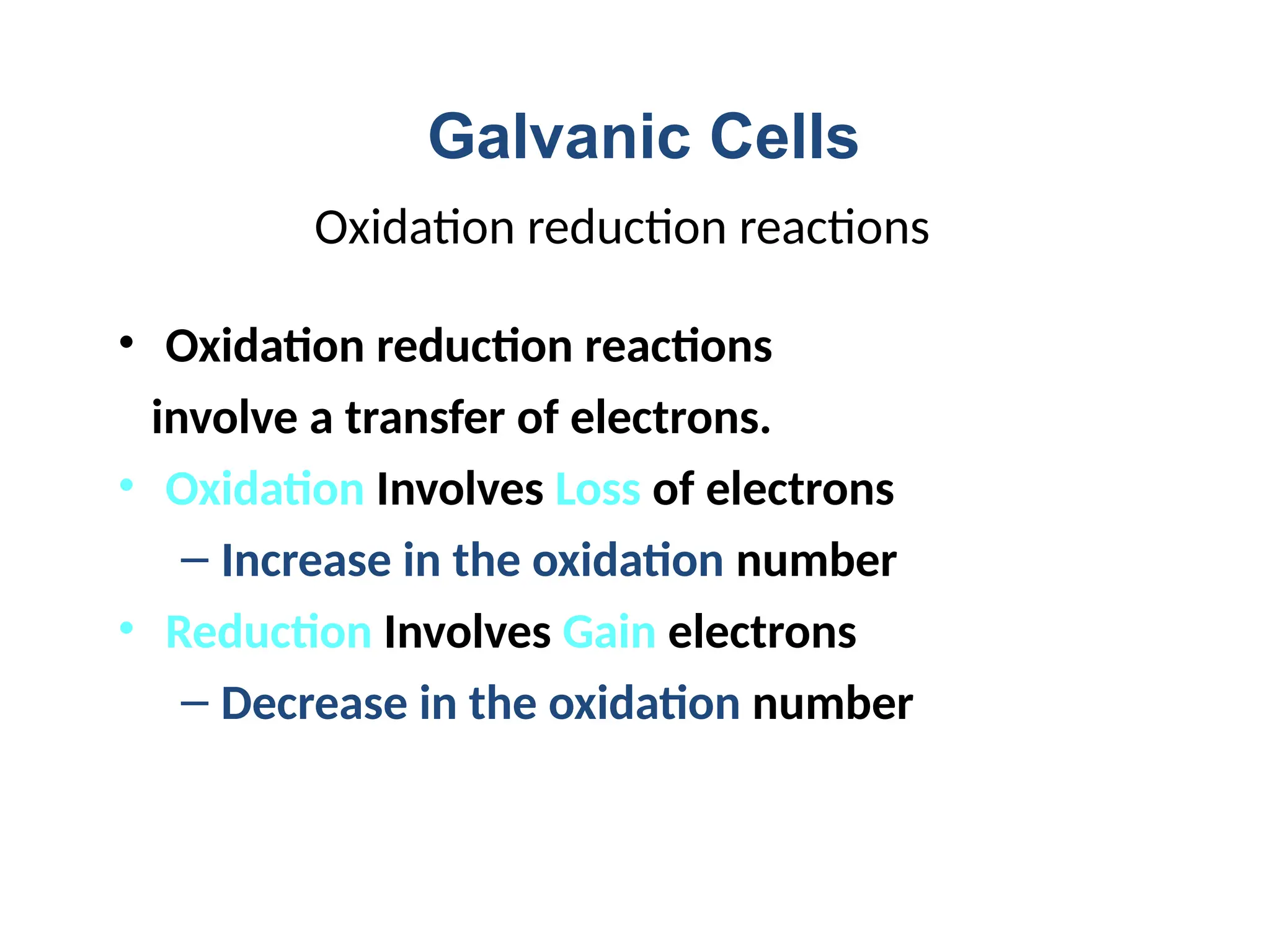
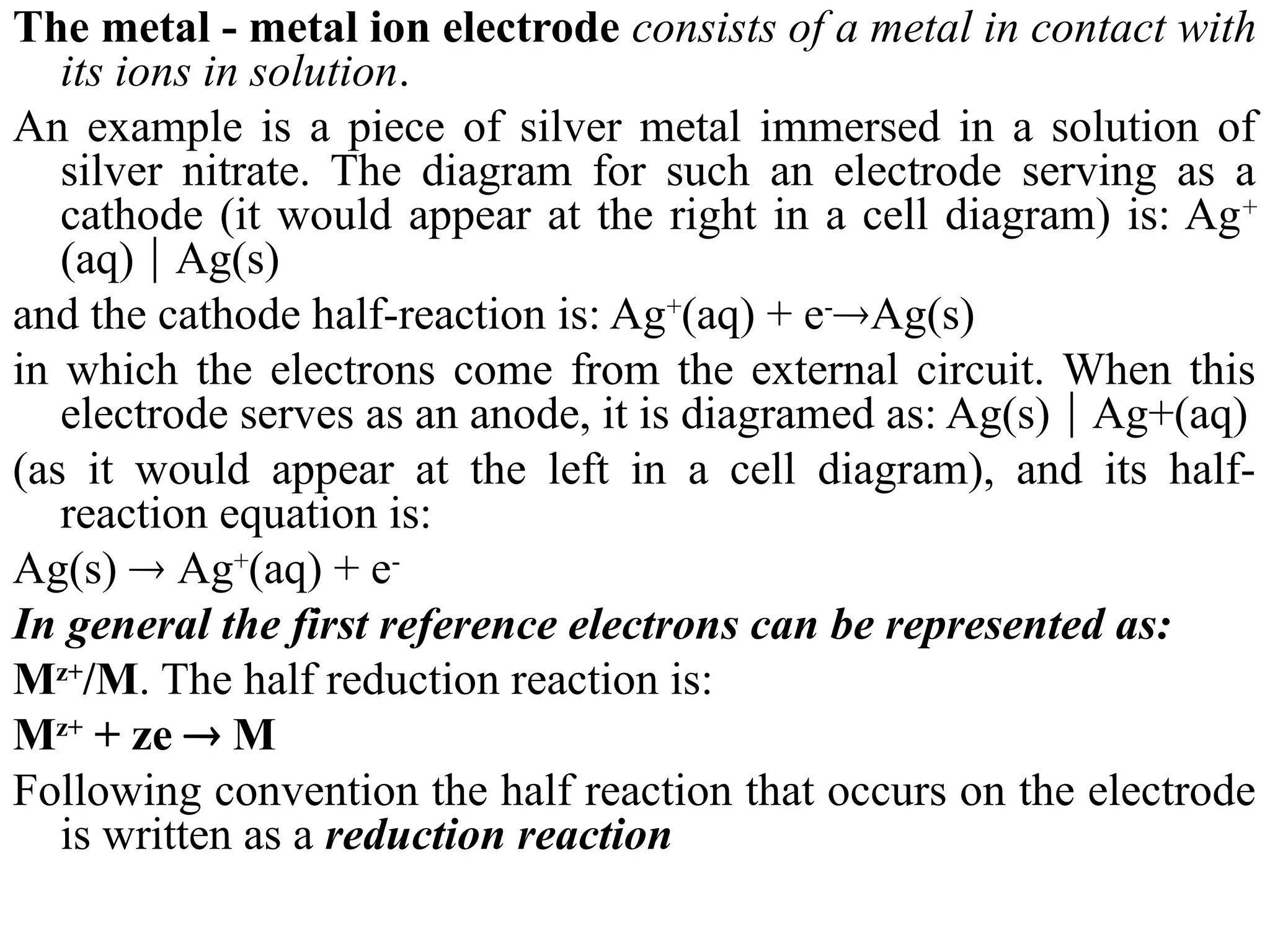
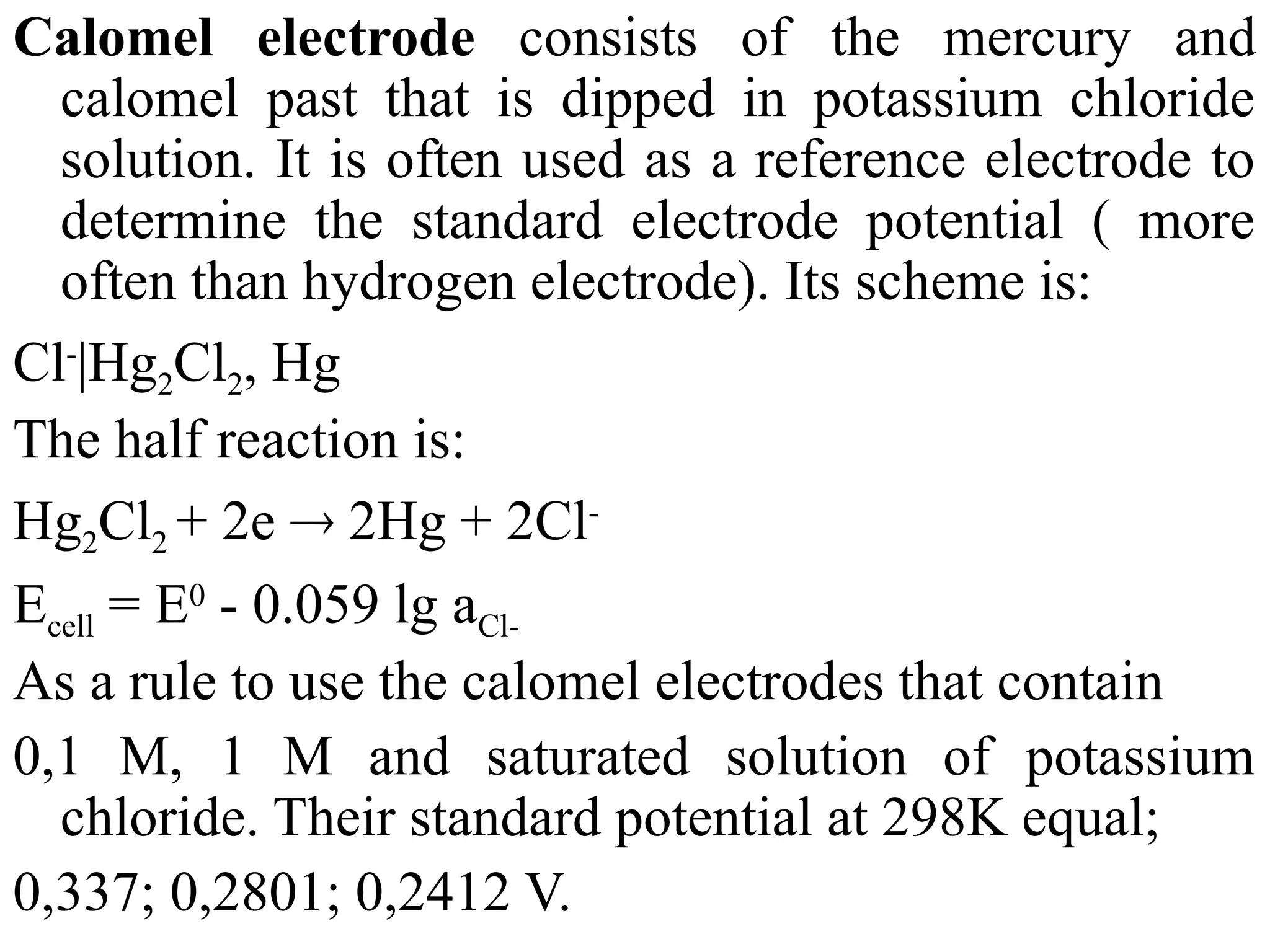
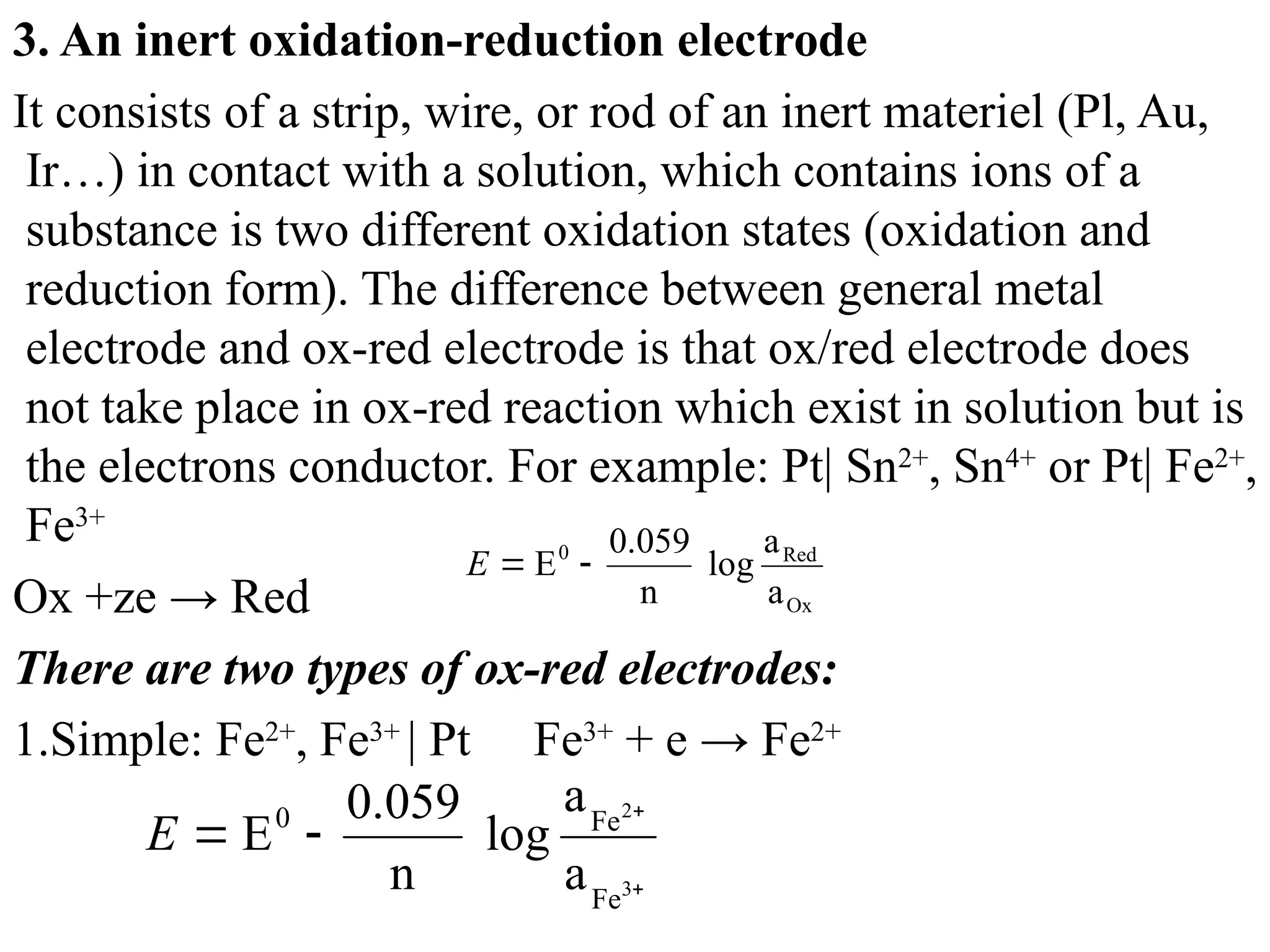
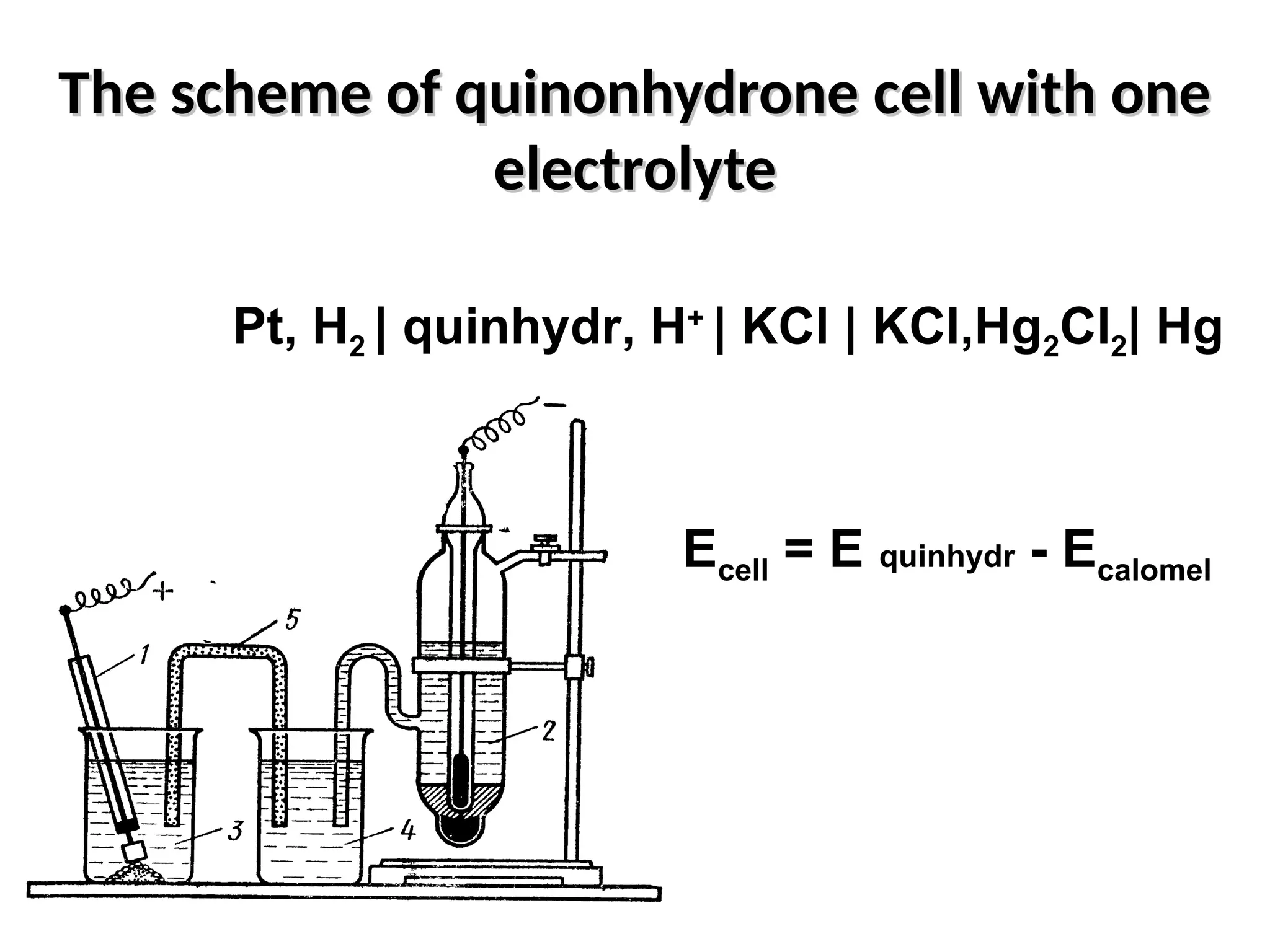
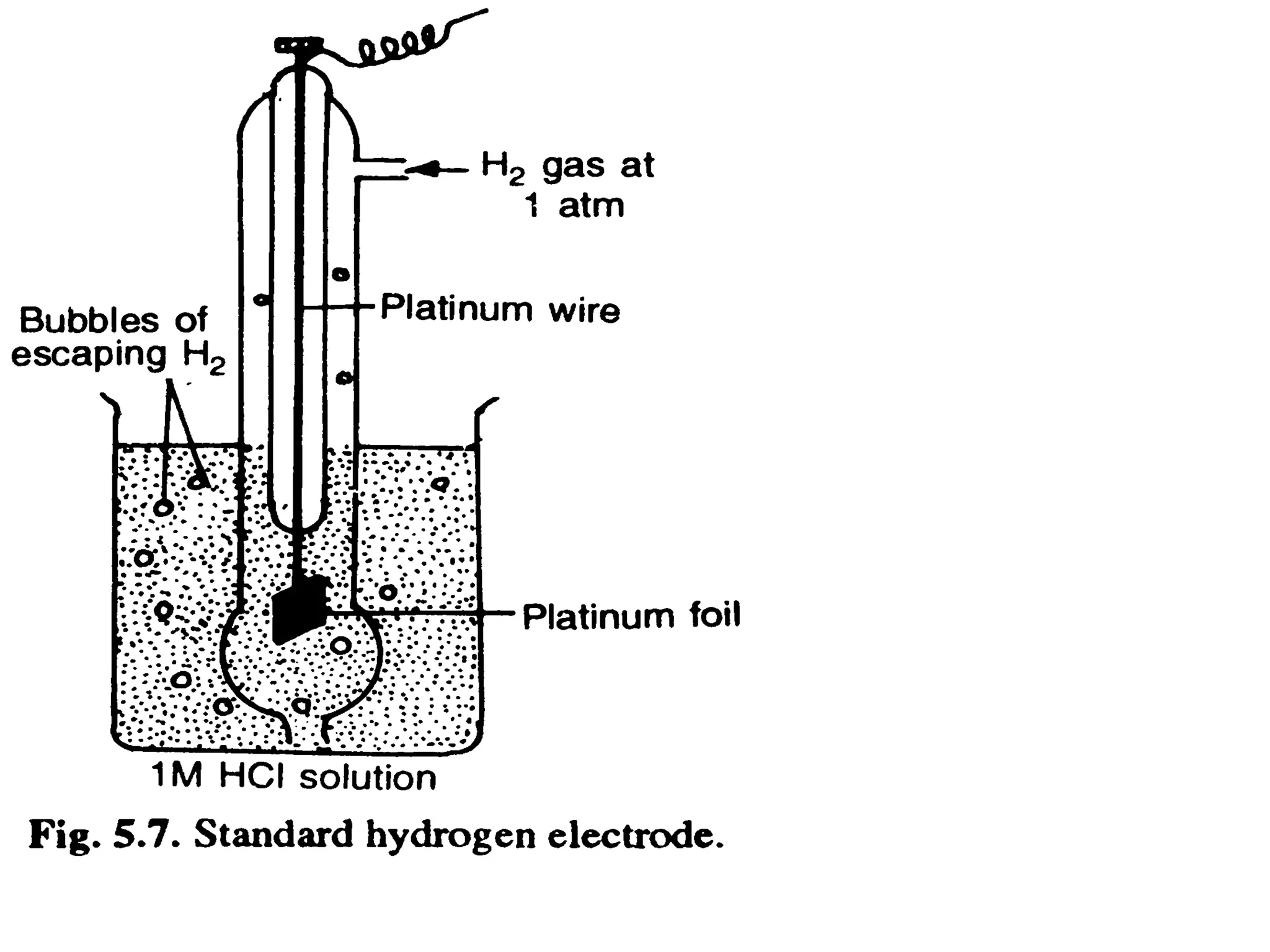
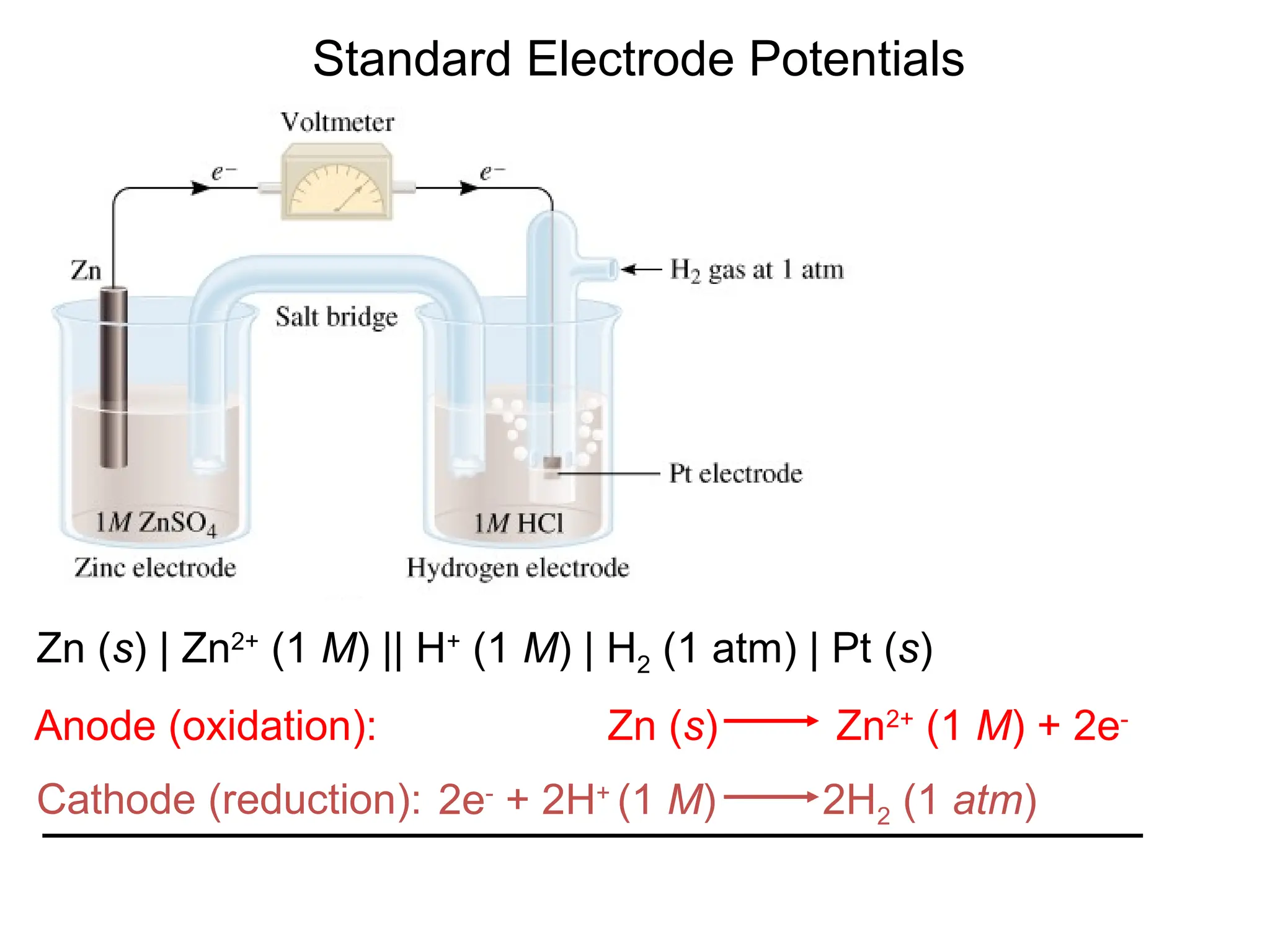
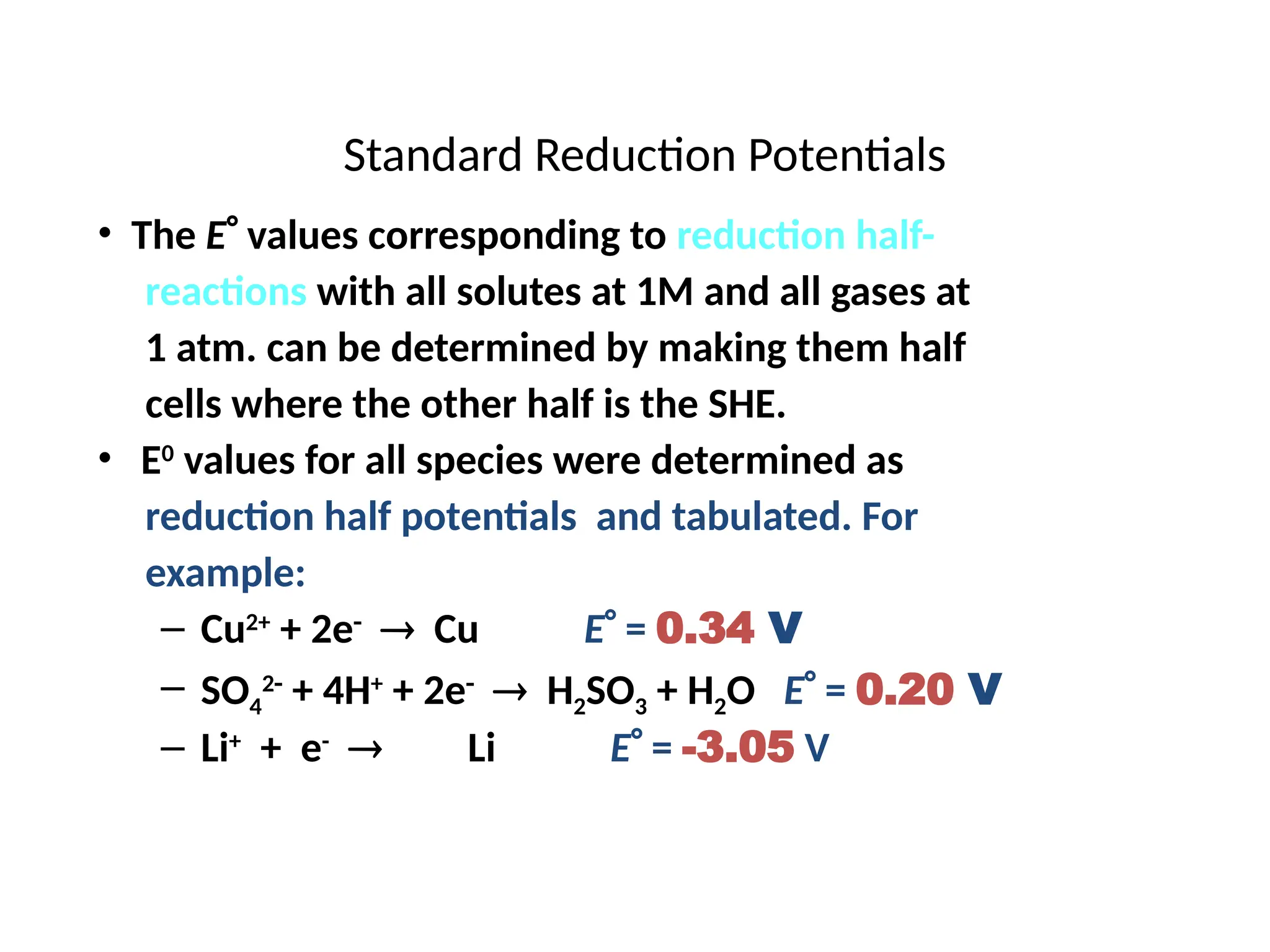
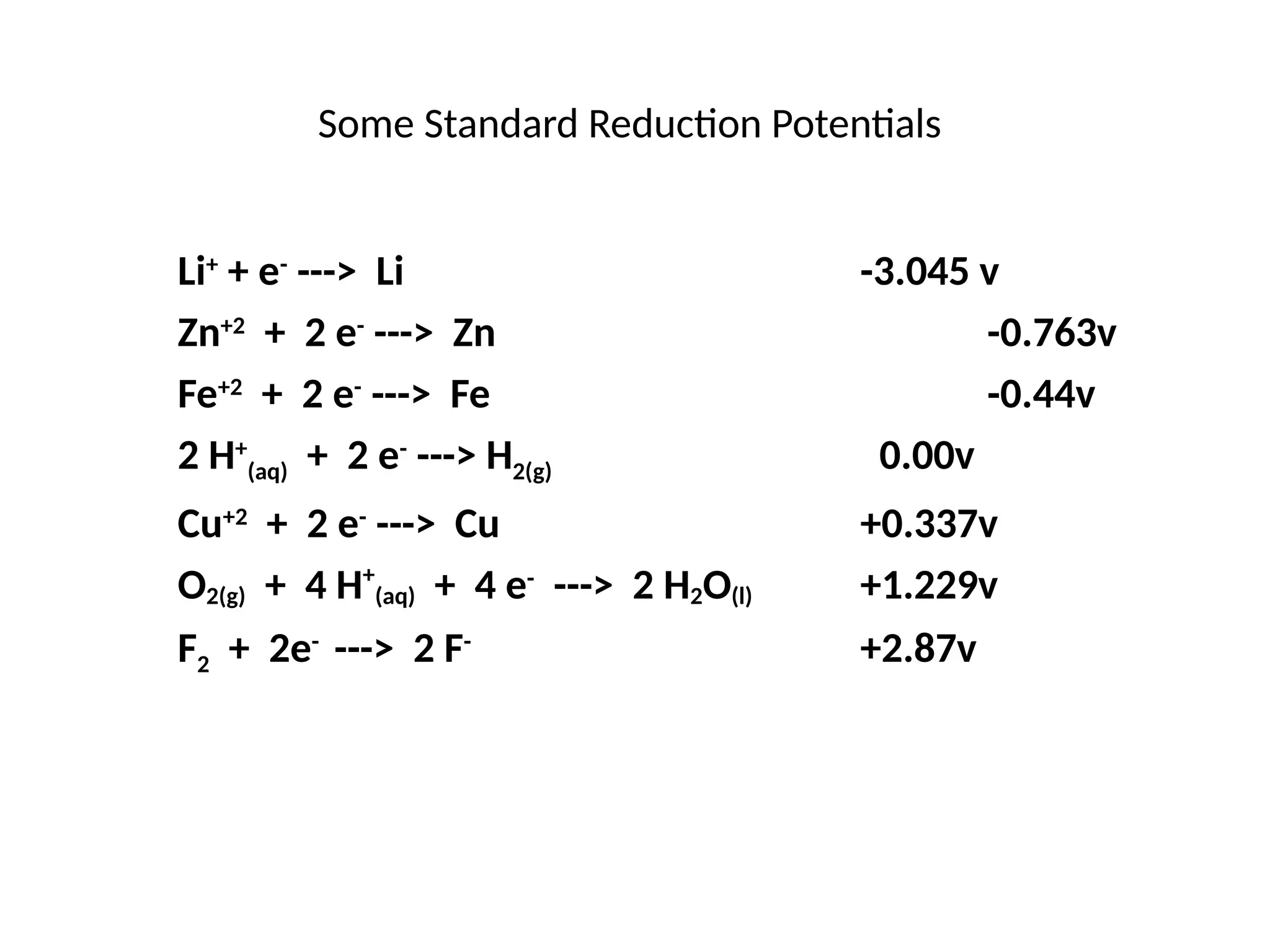
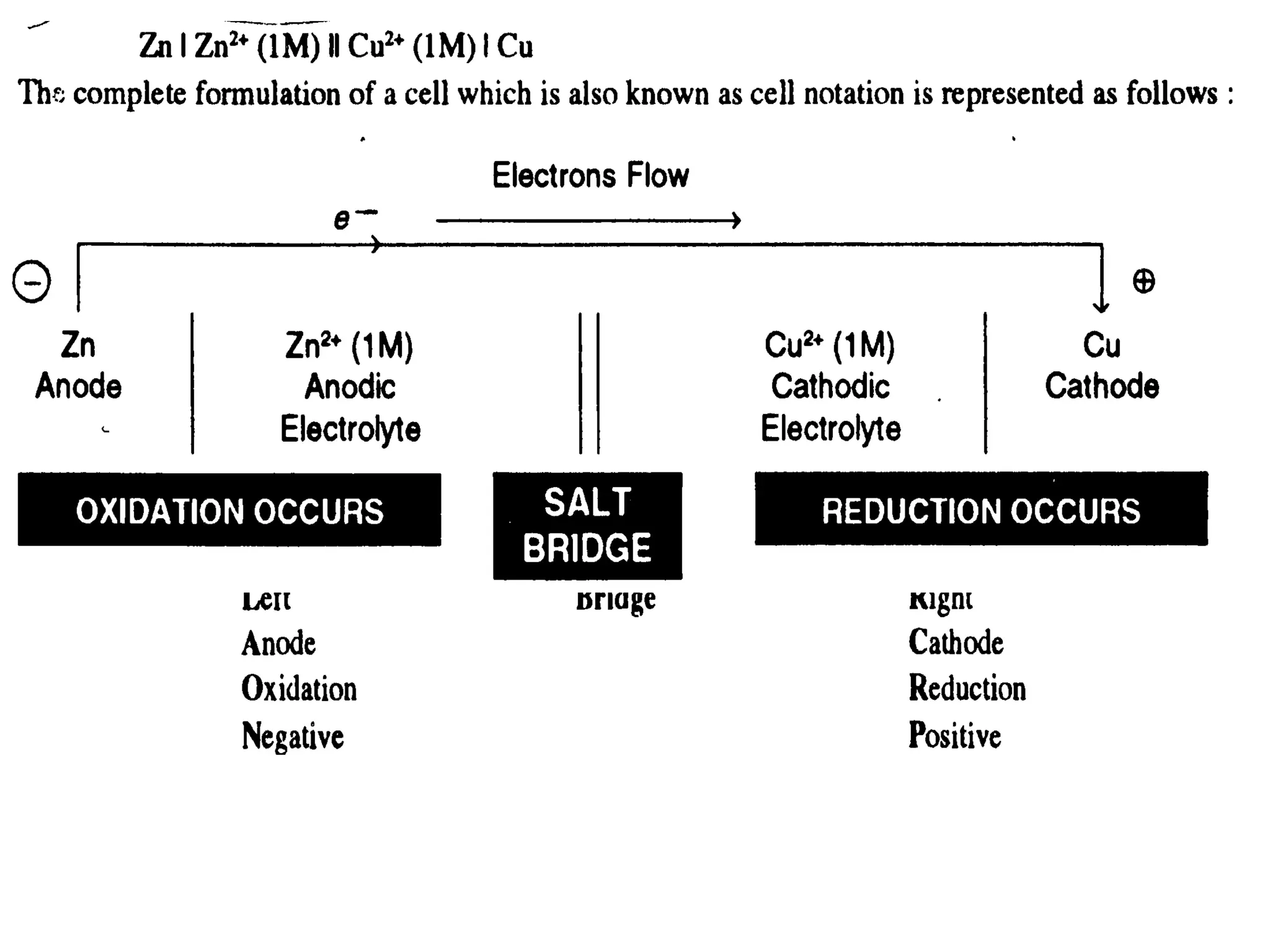
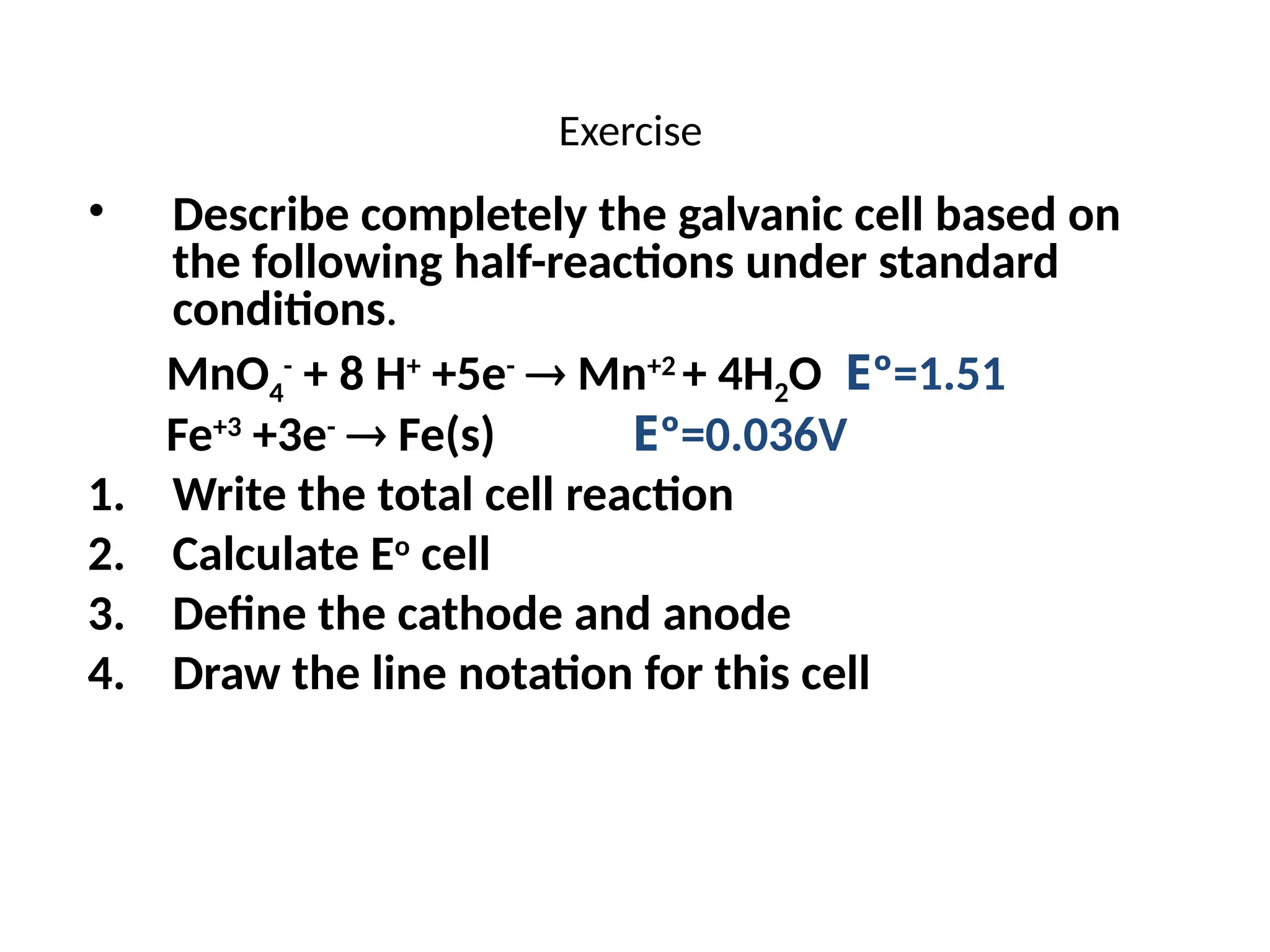
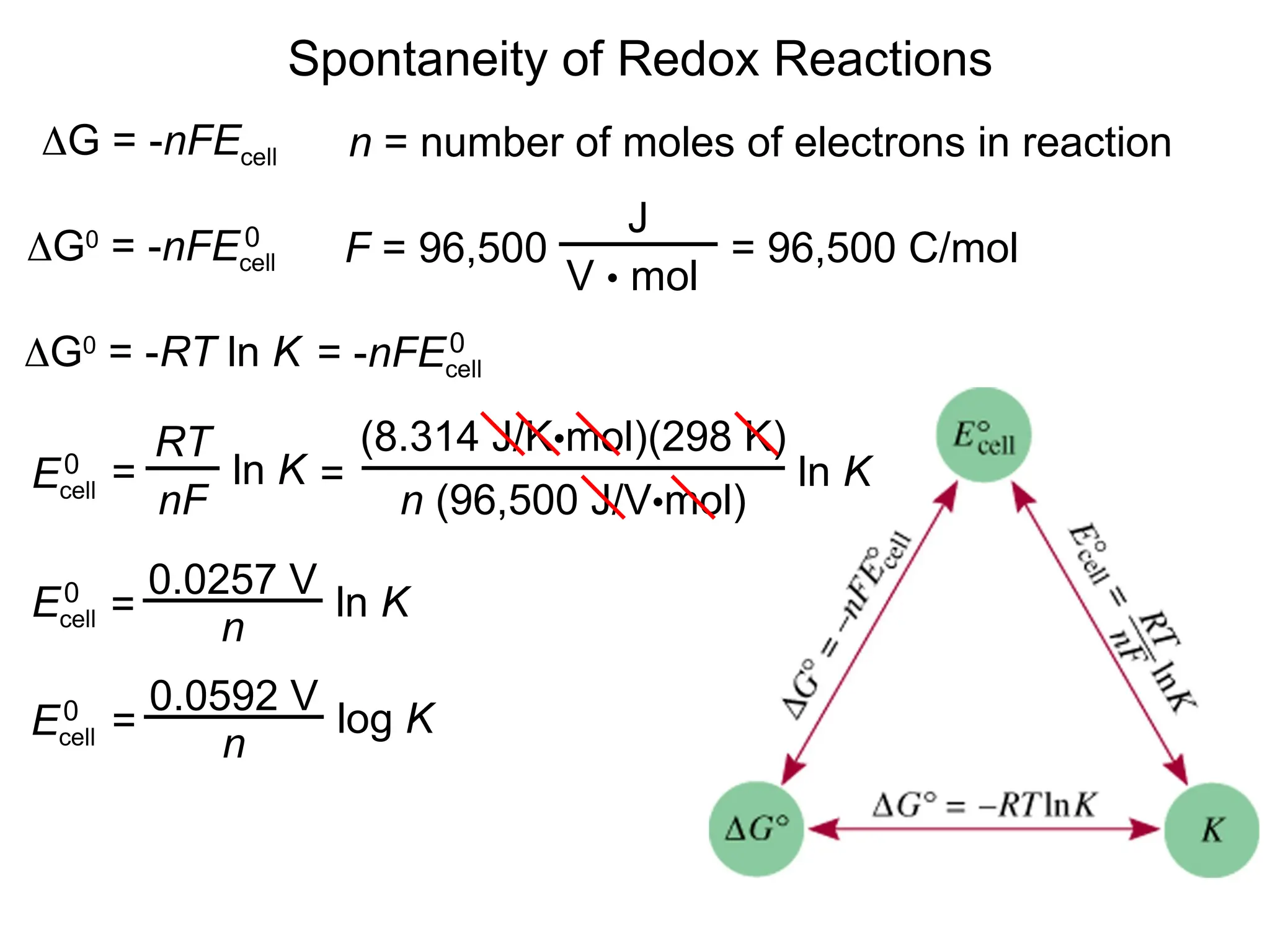
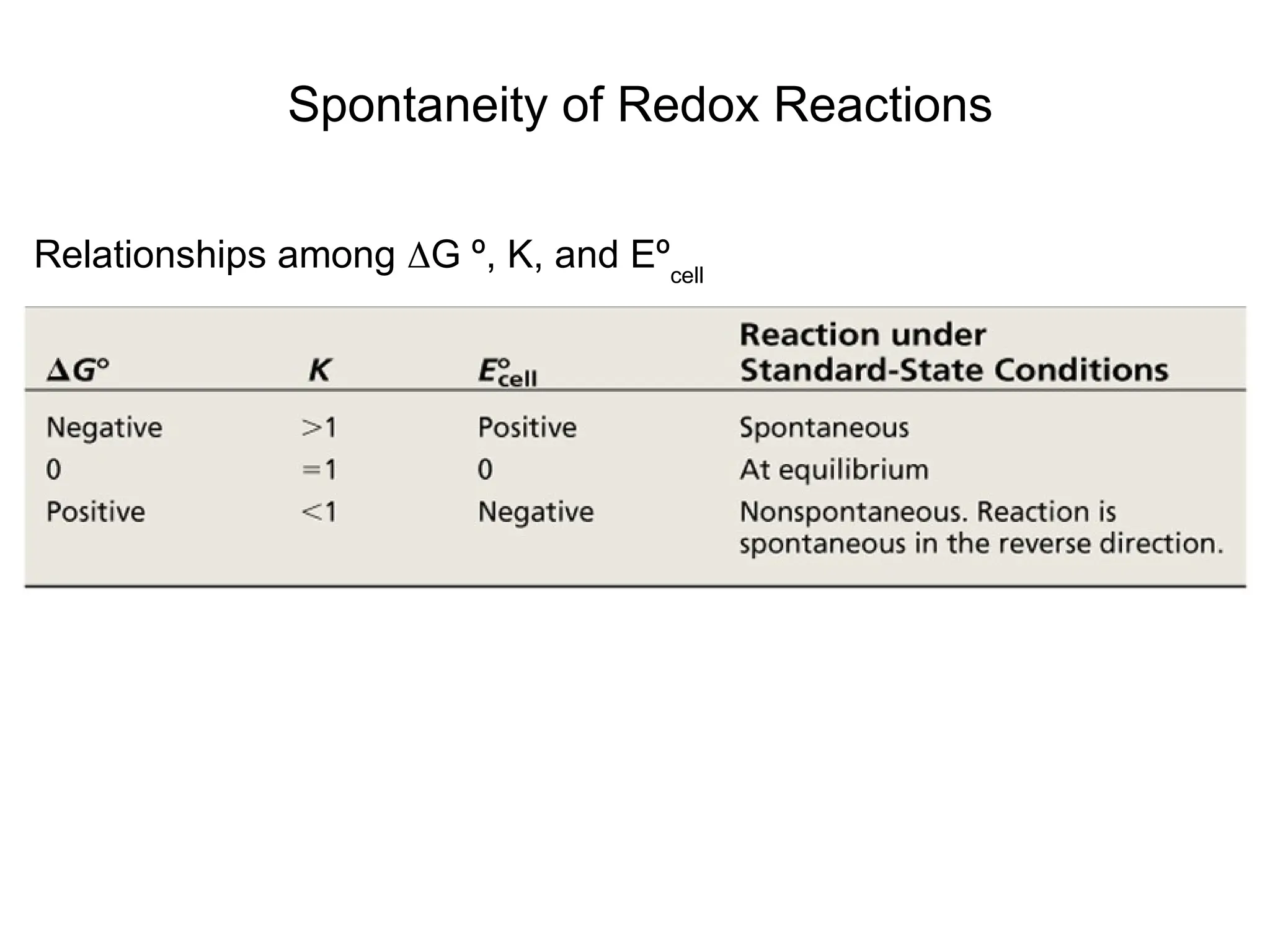
The document discusses electrochemistry, focusing on oxidation-reduction reactions, galvanic and electrolytic cells, and standard reduction potentials. It outlines the concepts of oxidation numbers, half-reactions, and the roles of oxidizing and reducing agents in redox processes. Additionally, it explains the mechanisms of electrochemical cells, including the importance of reference electrodes and how to calculate cell potential.











































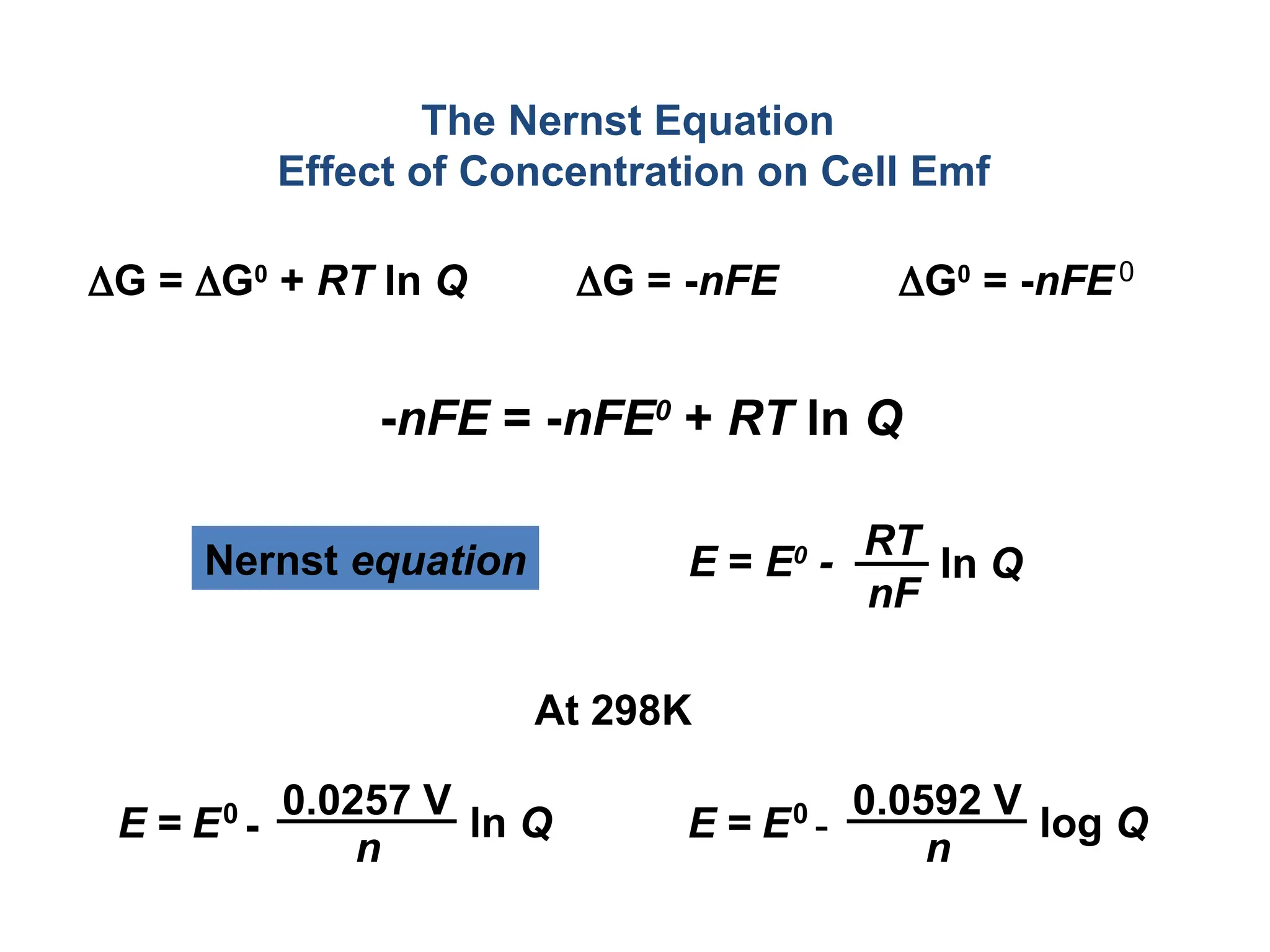

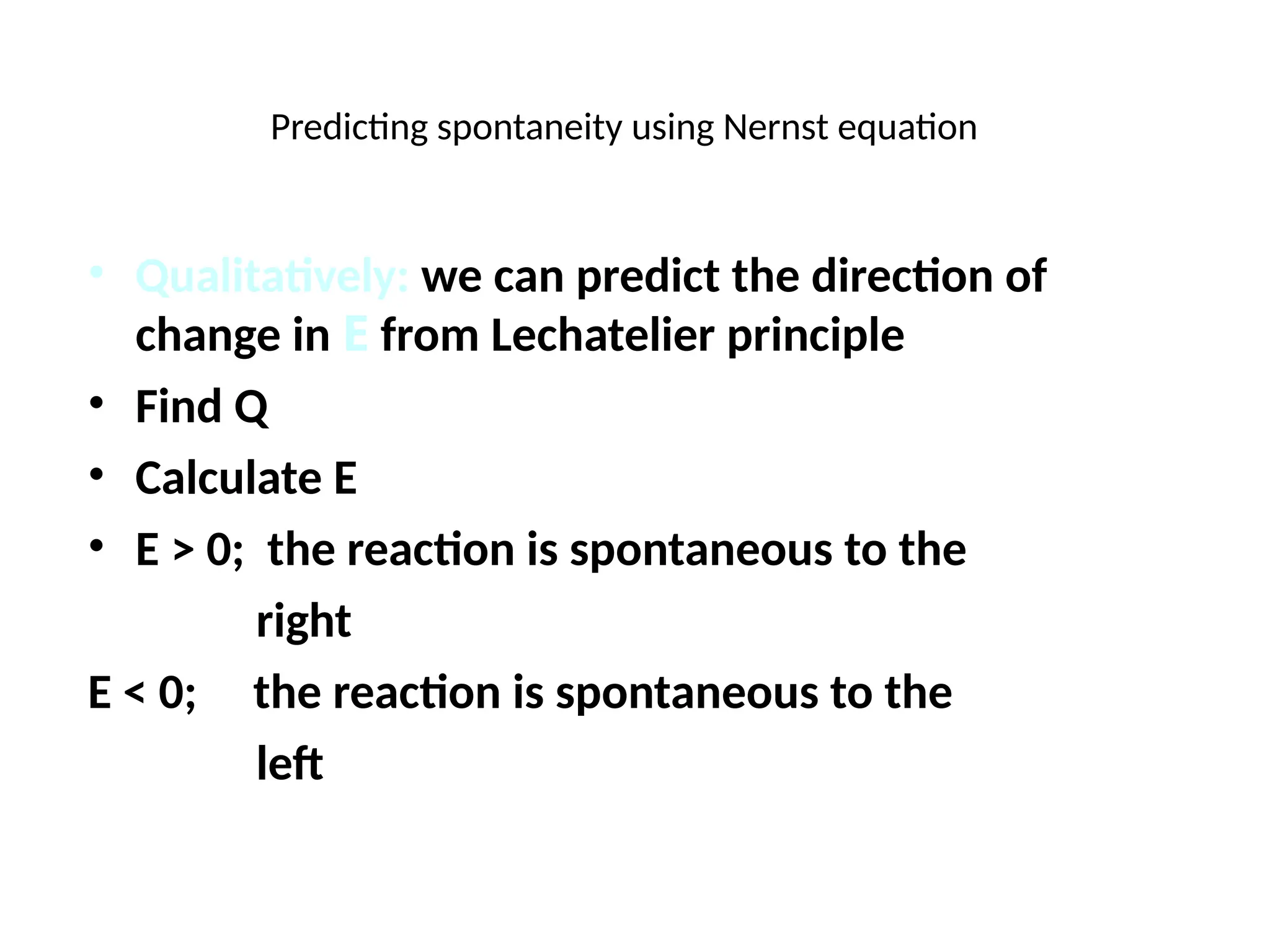
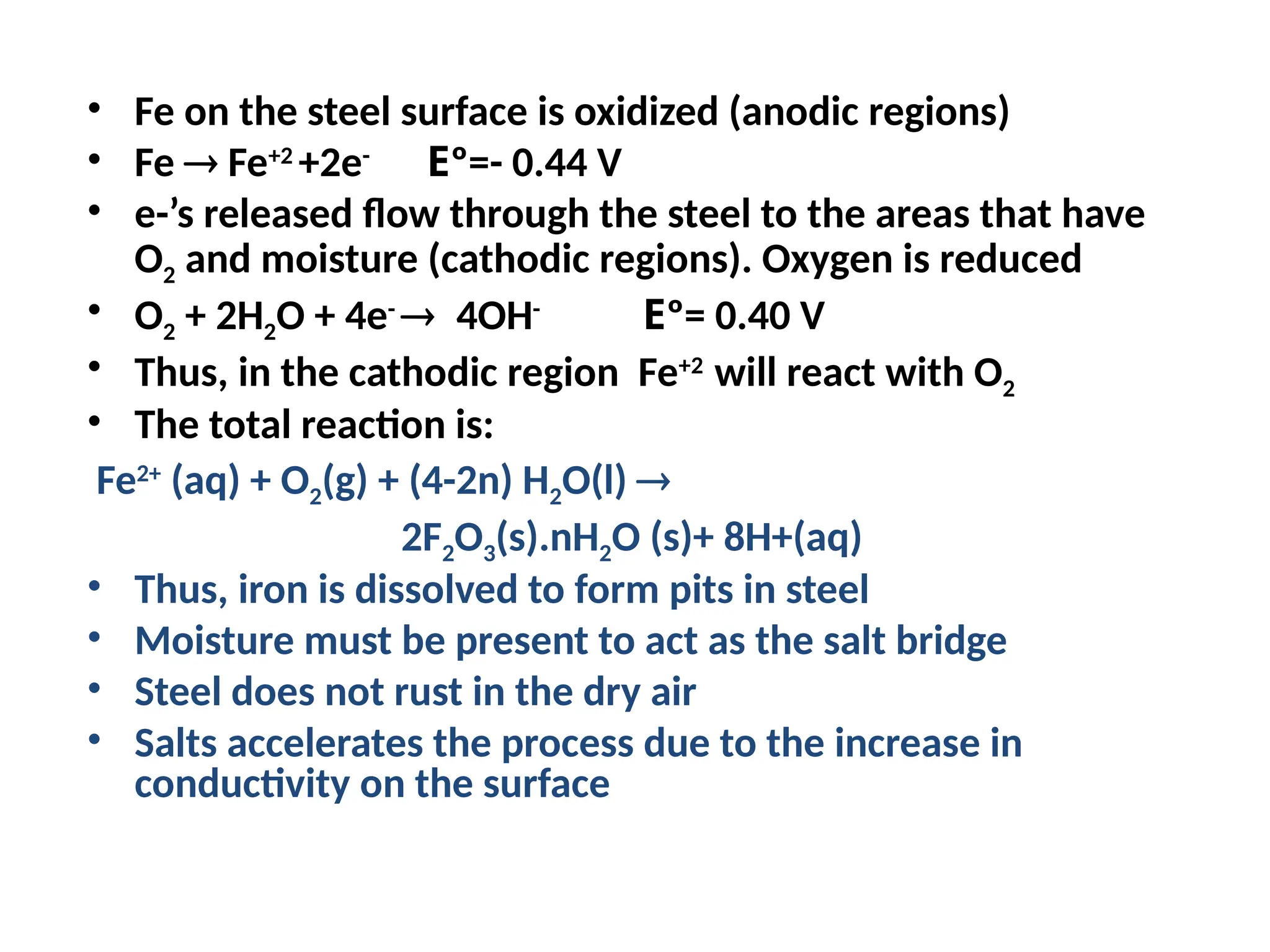
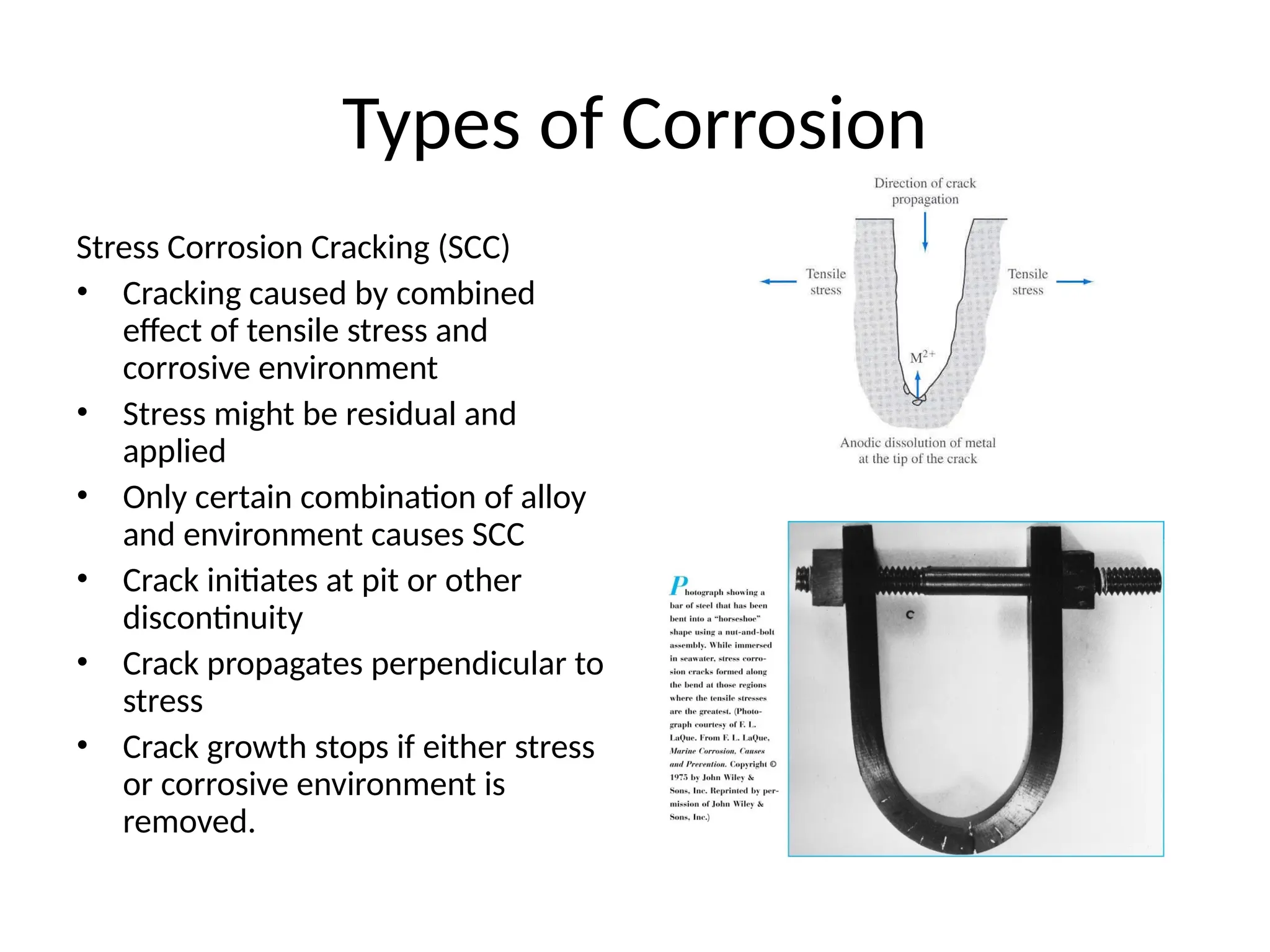
![Will the following reaction occur spontaneously at 250
C if
[Fe2+
] = 0.60 M and [Cd2+
] = 0.010 M?
Fe2+
(aq) + Cd (s) Fe (s) + Cd2+
(aq)
2e-
+ Fe2+
2Fe
Cd Cd2+
+ 2e-
Oxidation:
Reduction:
n = 2
E0
= -0.44 + (+0.40)
E0
= -0.04 V
E0
= EFe /Fe + ECd /Cd 2+
0 0
2+
-
0.0257 V
n
ln Q
E0
E =
-
0.0257 V
2
ln
-0.04 V
E =
0.010
0.60
E = ____________
E ___ 0 ________________](https://image.slidesharecdn.com/physicaliii-250206122233-17847206/75/Physical-Oxidation-numbers-Galvanic-cells-ppt-68-2048.jpg)
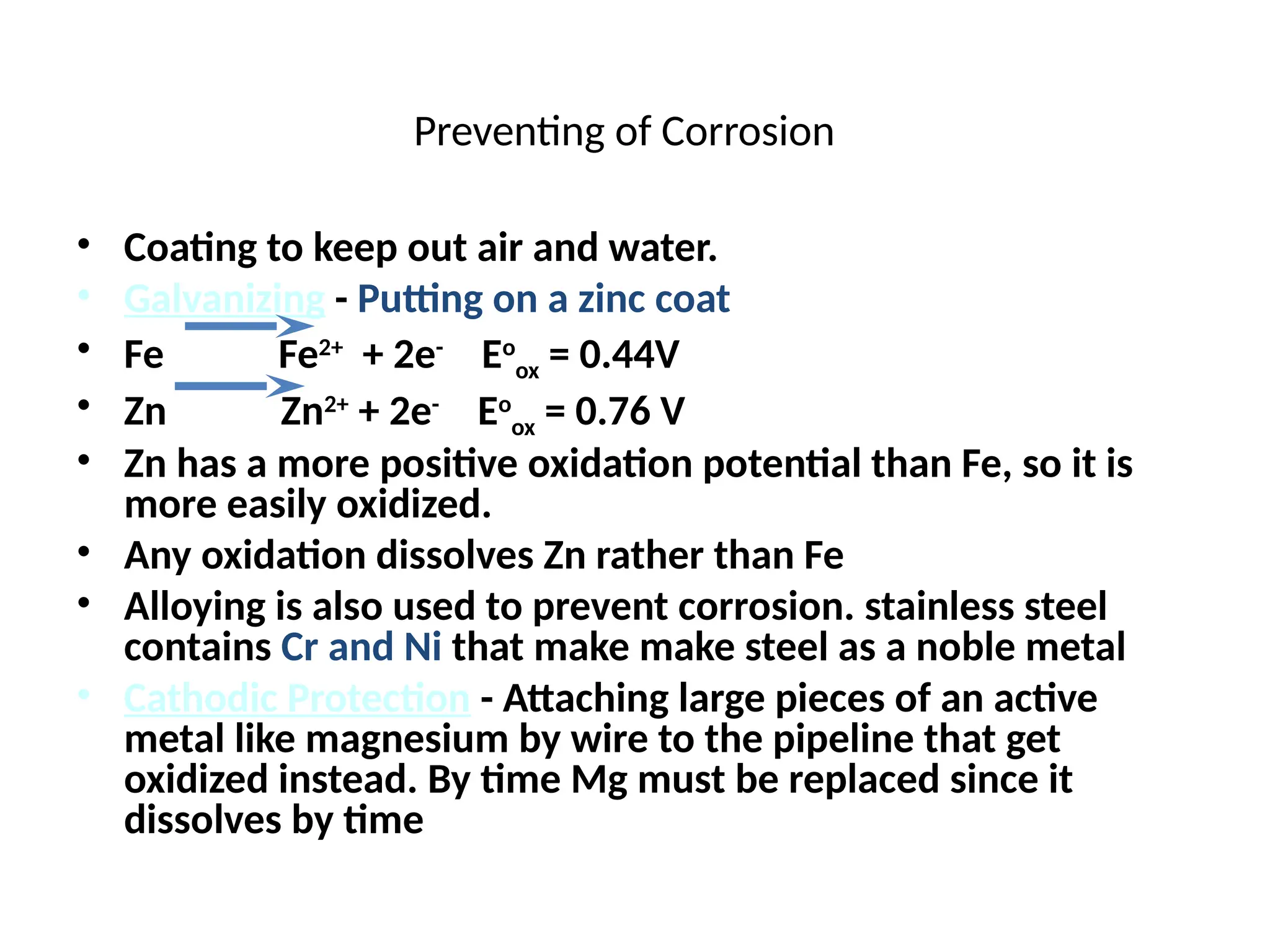
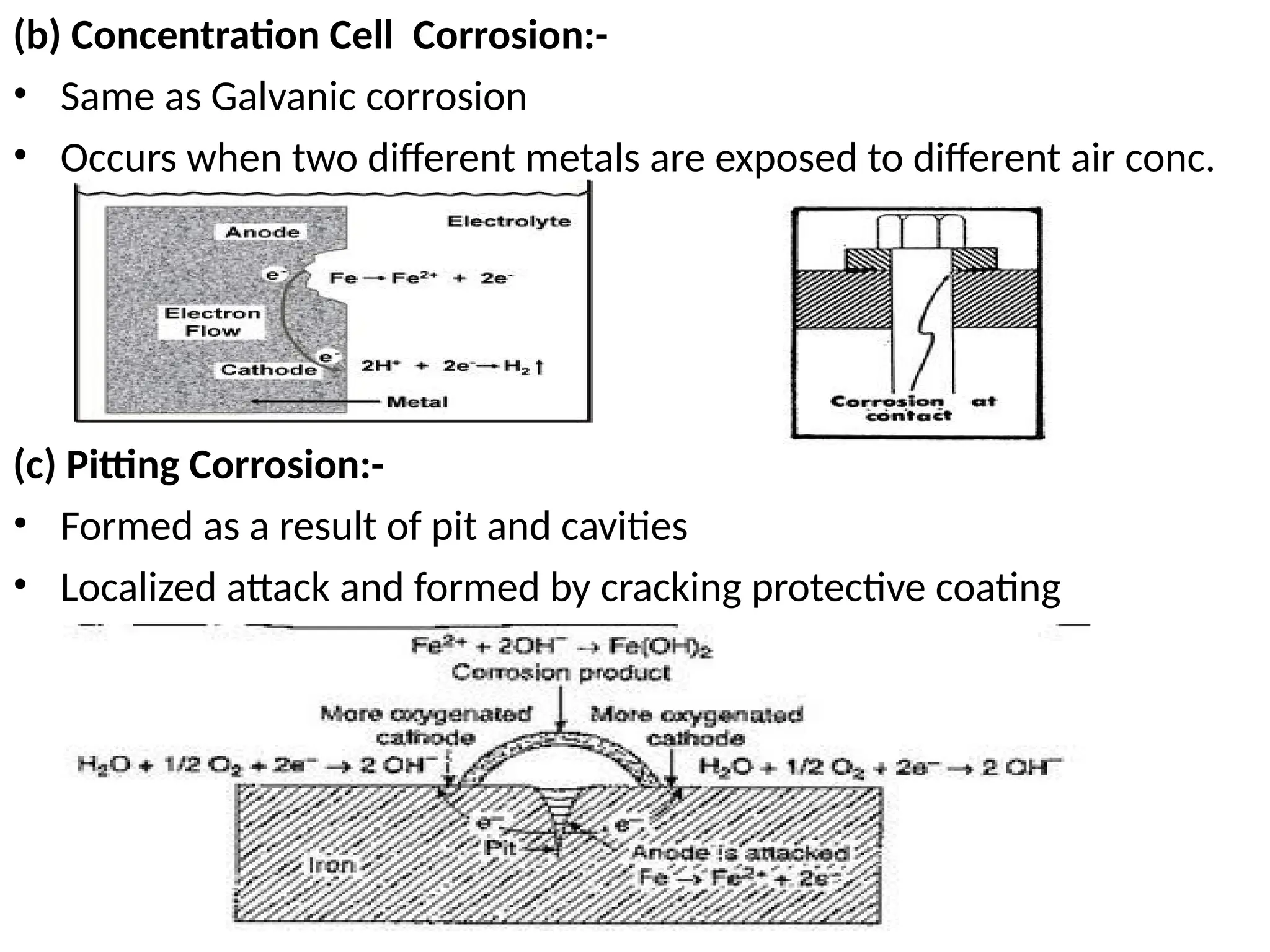
![Exercise
• Determine the cell potential at 25o
C for the following cell,
given that
• 2Al(s) + 3Mn+2
(aq) 2Al+3
(aq) + 3Mn(s)
[Mn2+
] = 0.50 M; [Al3+
]=1.50 M; E0
cell = 0.4
• Always we have to figure out n from the balanced
equation
2(Al(s)
+
Al+3
(aq) + 3e-
)
3(Mn+2
(aq) + 2e-
Mn(s))
n = 6
-
0.0592 V
n
log Q
E0
E =](https://image.slidesharecdn.com/physicaliii-250206122233-17847206/75/Physical-Oxidation-numbers-Galvanic-cells-ppt-69-2048.jpg)
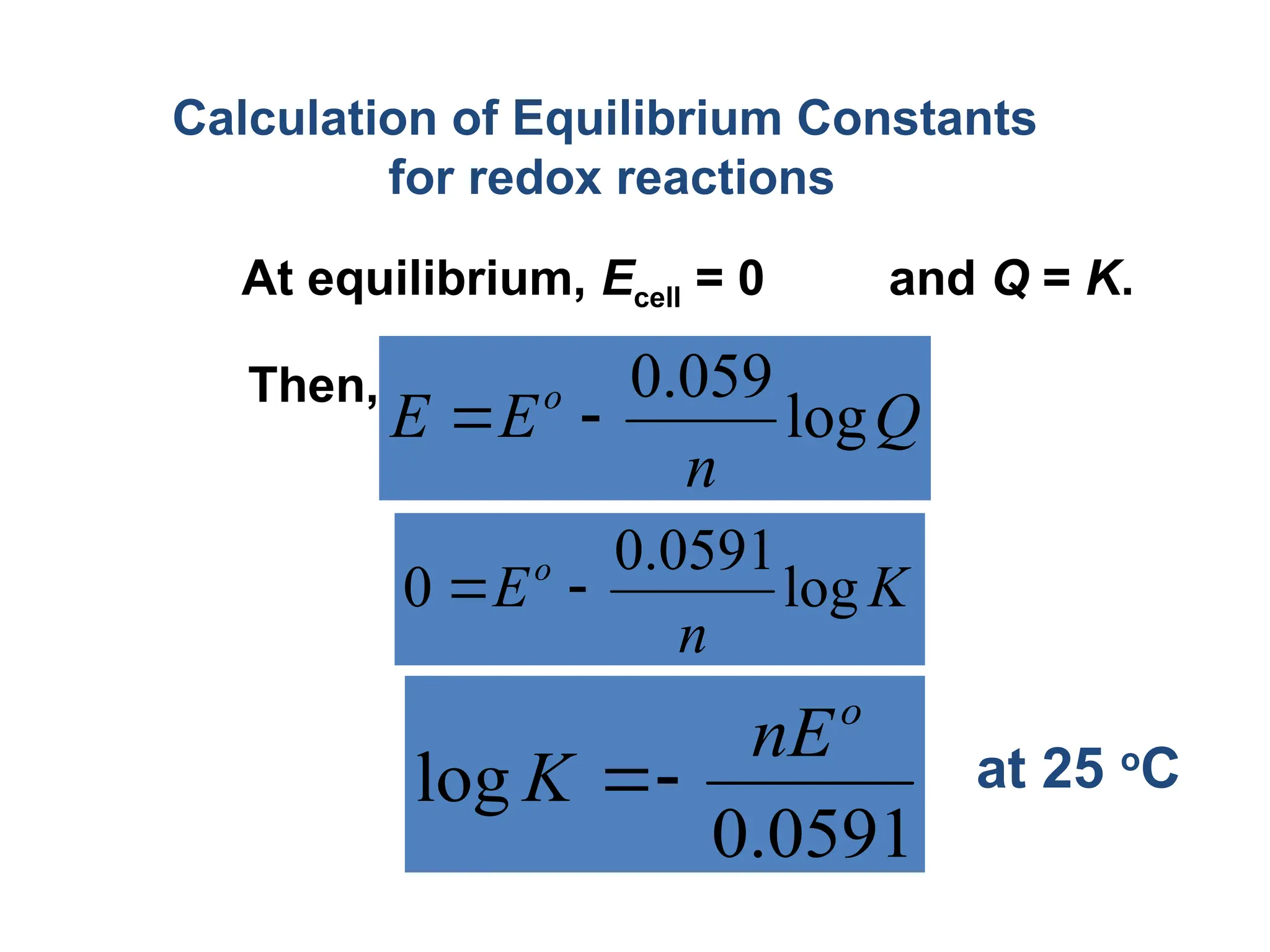

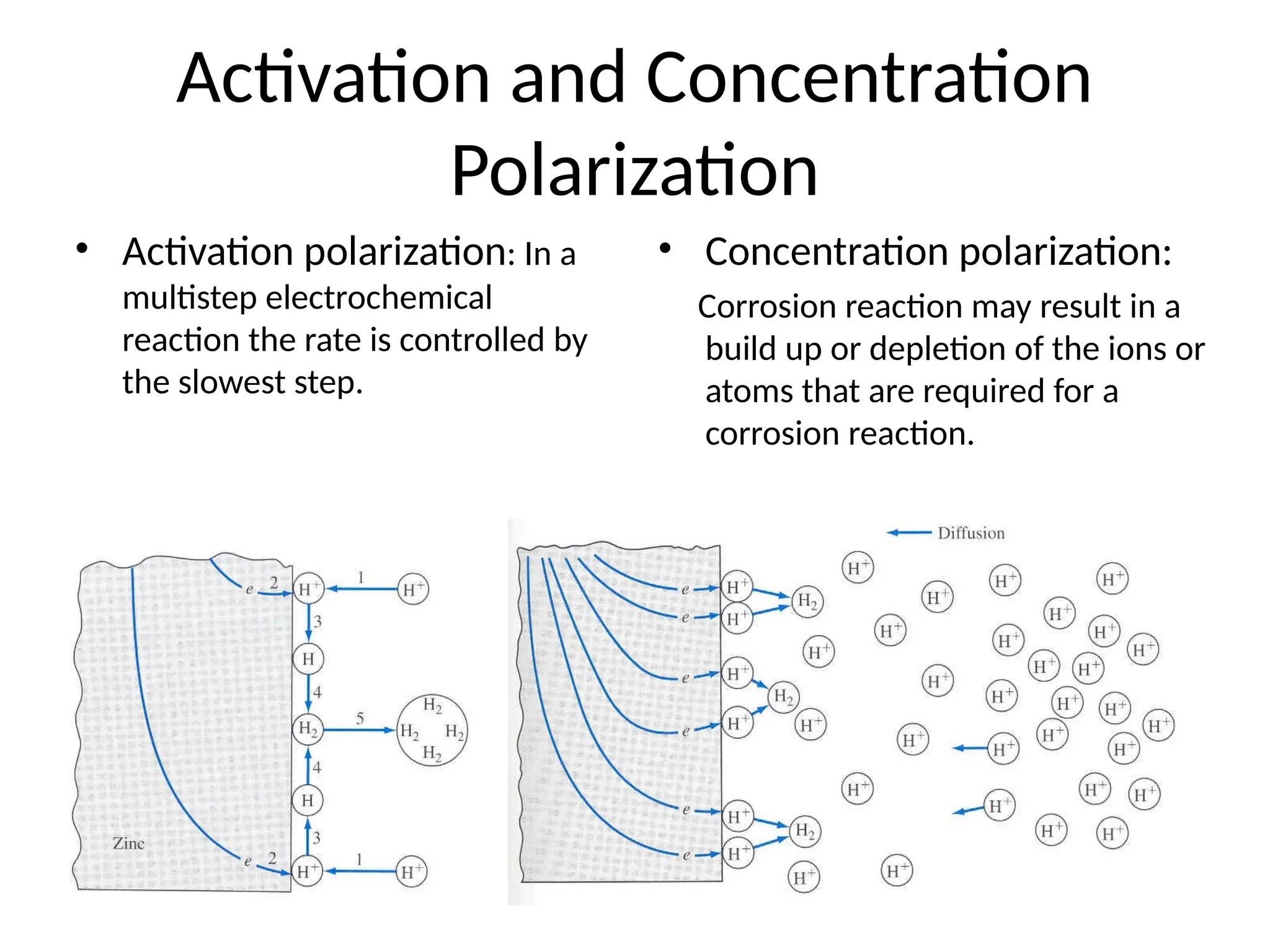
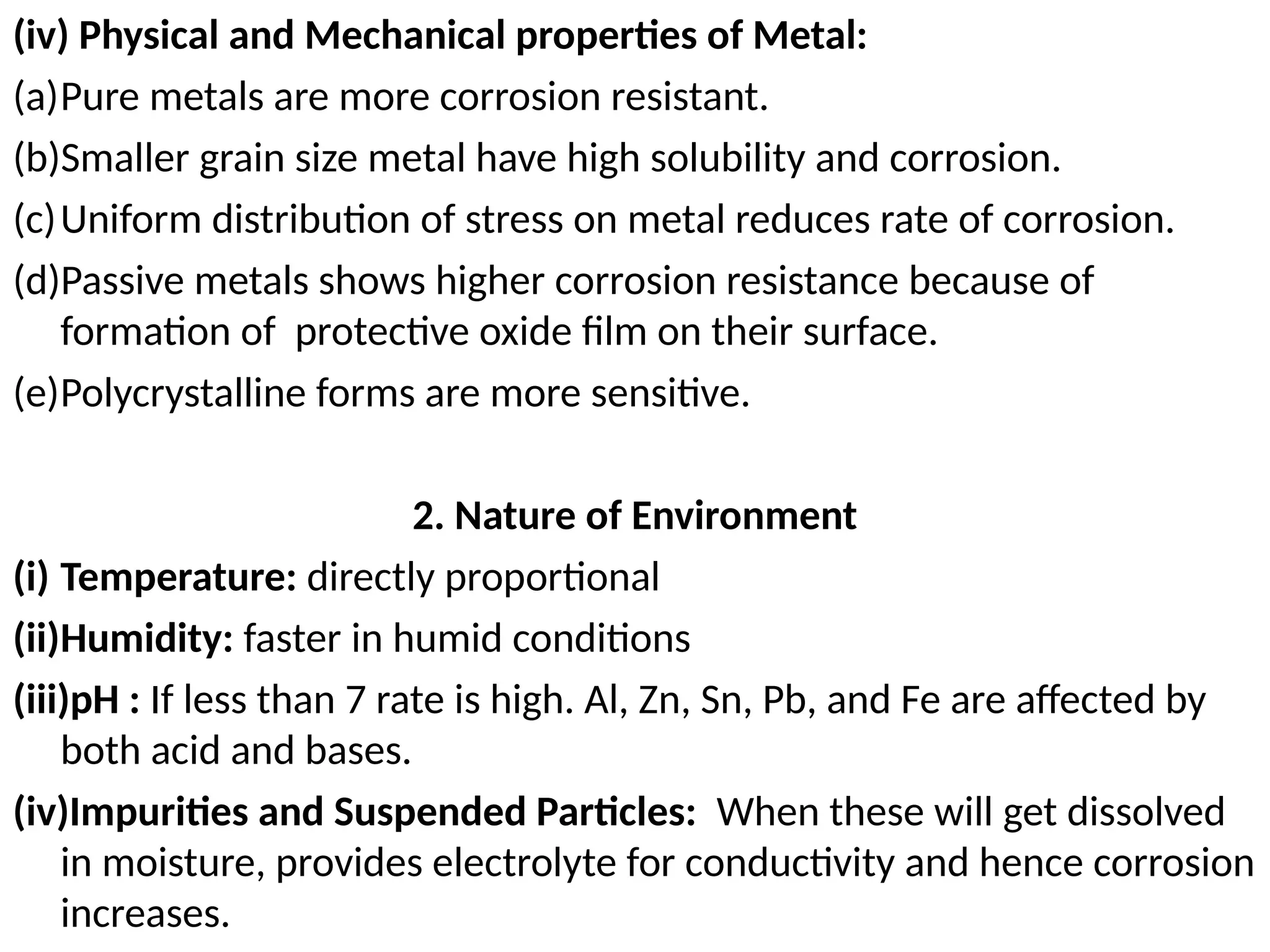
![Concentration Cell: both compartments contain same
components but at different concentrations
Half cell potential are not
identical
Because the Ag+
Conc.
On both sides are not
same
Eright > Eleft
• To make them equal, [Ag+
]
On both sides should same
• Electrons move from left to
right](https://image.slidesharecdn.com/physicaliii-250206122233-17847206/75/Physical-Oxidation-numbers-Galvanic-cells-ppt-72-2048.jpg)