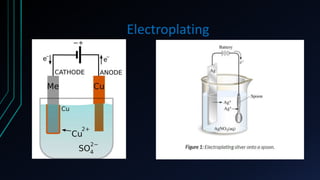
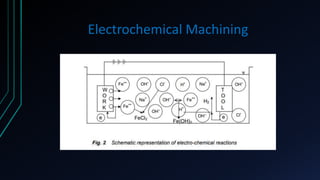
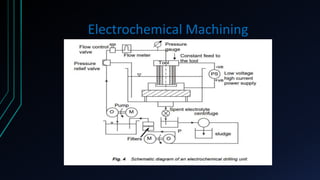
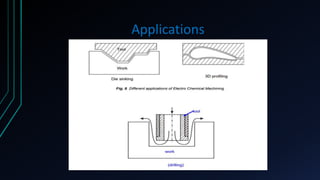
The document provides an overview of electrochemical machining (ECM), detailing its principles based on Faraday's laws of electrolysis, historical development, and comparison with electroplating. It highlights ECM's advantages, such as the ability to produce complex shapes without tool wear, as well as limitations including the requirement for electrically conductive materials. Applications of ECM include die sinking, profiling, drilling, and micro-machining.