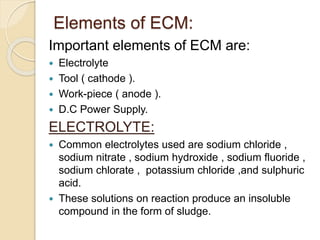
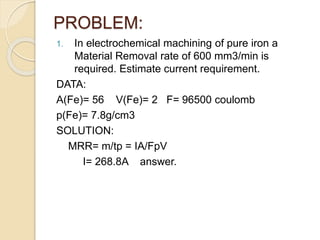
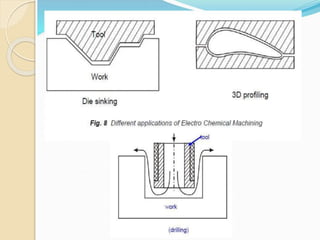
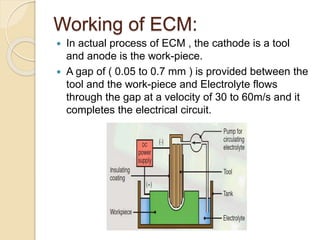
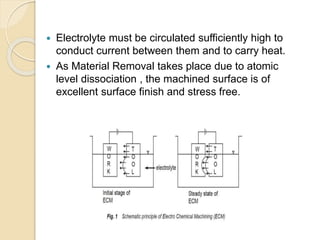
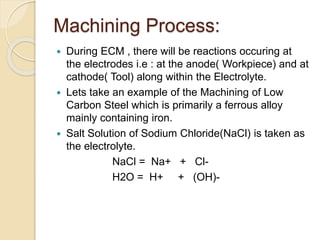
Electrochemical machining (ECM) uses electrolysis to remove metal where the workpiece acts as the anode. A power supply creates a potential difference between the cathode tool and the workpiece in an electrolyte solution, which causes a chemical reaction that dissolves the workpiece metal atom by atom. ECM can machine harder metals than possible with conventional machining and produces a smooth, non-distorted surface. However, it requires high energy and can only machine electrically conductive materials.


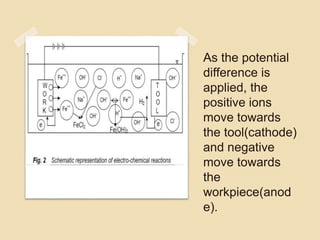
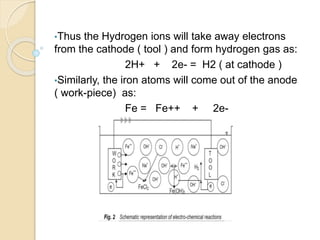
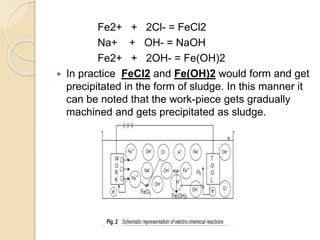
![ Within the electrolyte iron ions would combine with
chloride to form Ferrous Chloride (FeCl2 ) .
Hydroxyl ions to form Ferrous Hydroxide
[Fe(OH)2] .
Similarly Sodium ions would combine with Hydroxyl
ions to form Sodium Hydroxide [Na(OH)].](https://image.slidesharecdn.com/ecm-180323133947/85/ECM-10-320.jpg)