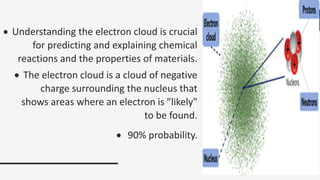
Electron theory aims to explain the structure and properties of matter through its electronic structure. It states that all matter is comprised of molecules made up of atoms, which contain protons, neutrons, and electrons. Electrons play a key role in electricity as their movement constitutes electric current. Within atoms, electrons exist in specific energy levels or shells around the nucleus, and their behavior is described by quantum mechanics.