1. DNA is hydrolyzed by acids below pH 7 via an SN1 reaction mechanism where the acid attacks nucleophilic nitrogens, especially N7 of guanine. This leads to depurination and complete hydrolysis at very low pH.
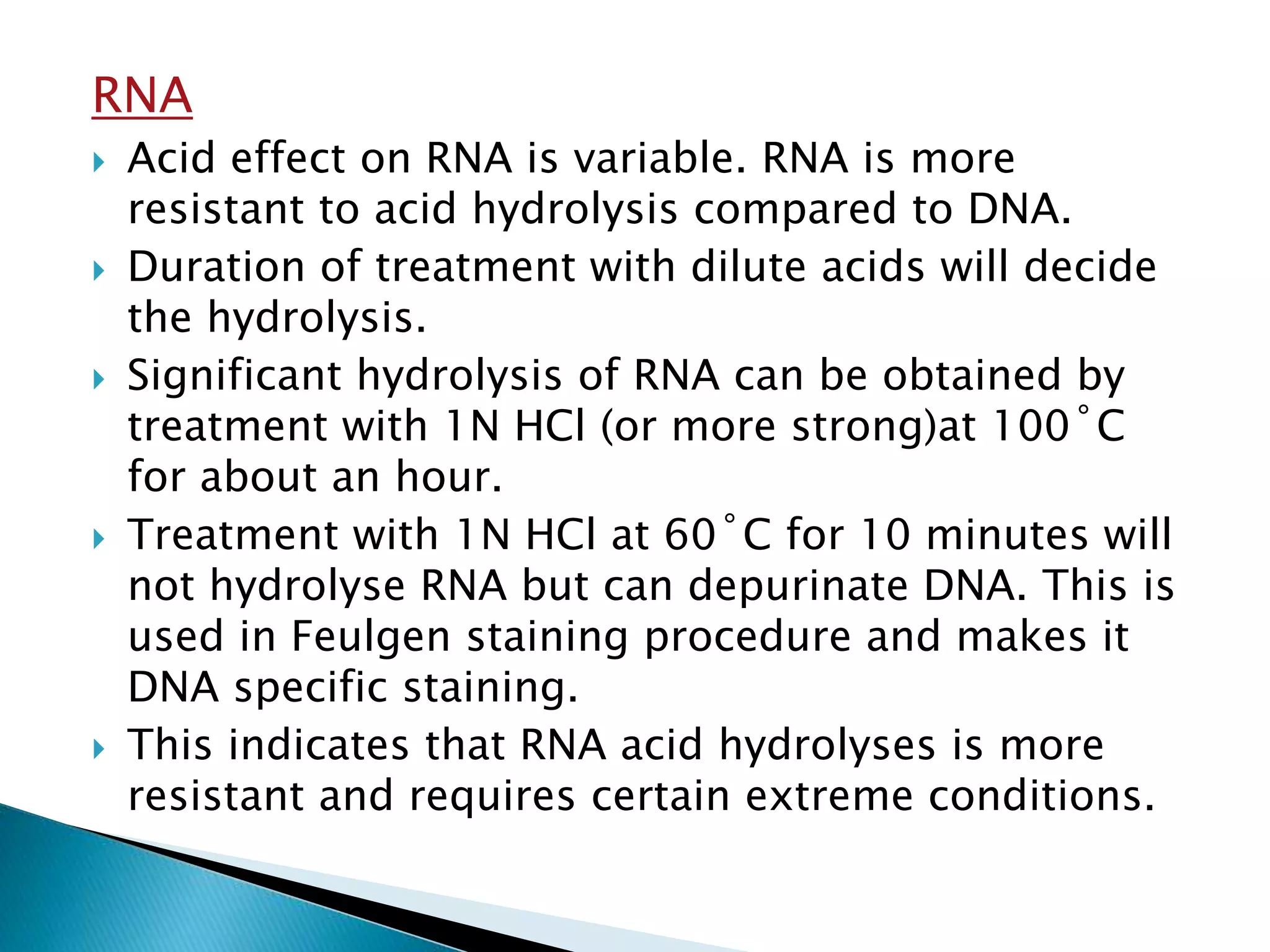
2. RNA is more resistant to acid hydrolysis than DNA but can still be hydrolyzed by stronger acids like 1N HCl at high temperatures. Acid treatment can depurinate DNA but not RNA.
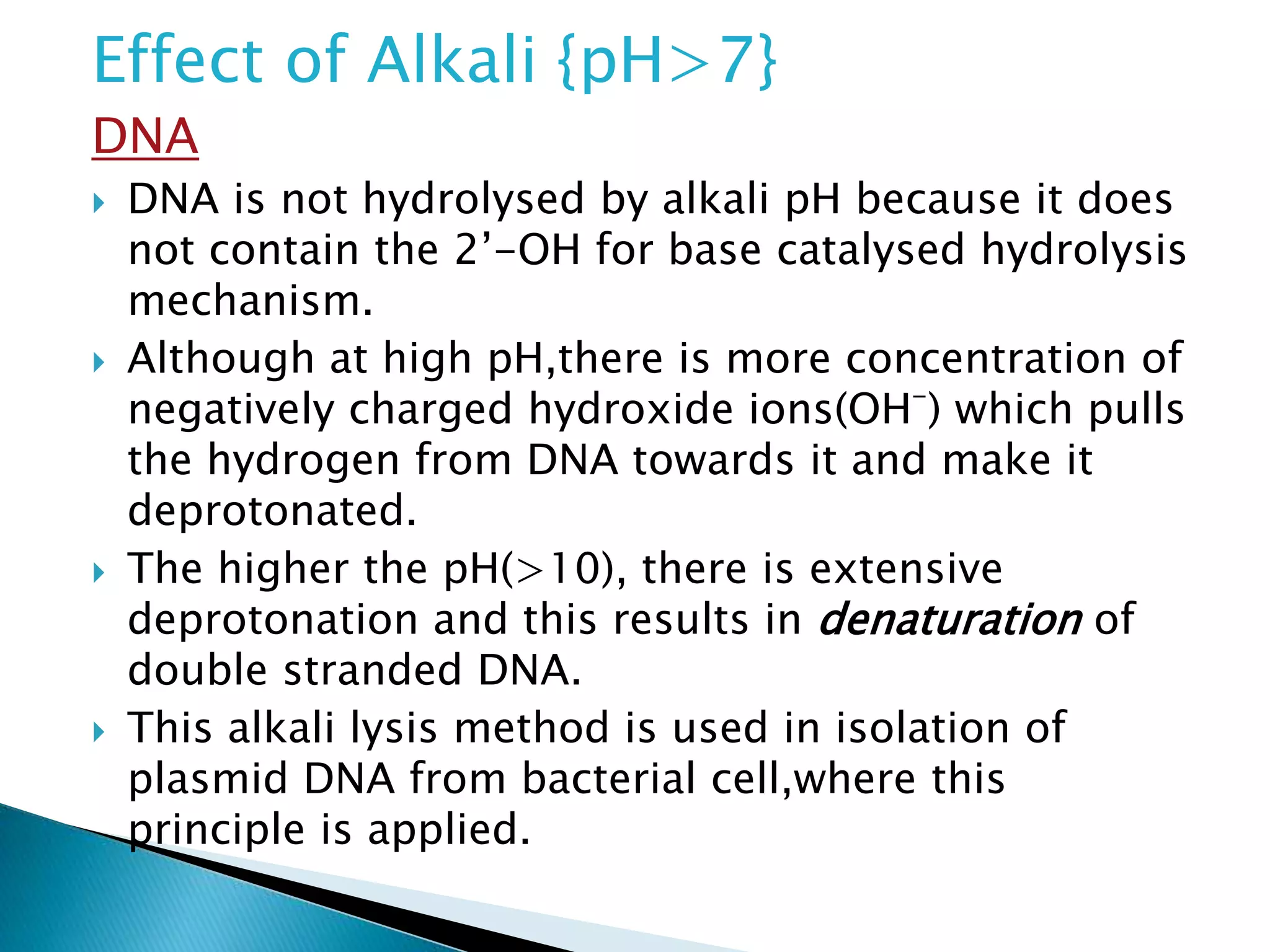
3. DNA is not hydrolyzed by alkalis above pH 7 because it lacks the 2'-OH group required for base-catalyzed hydrolysis. RNA contains this group and can be easily hydrolyzed by alkaline pH through a mechanism