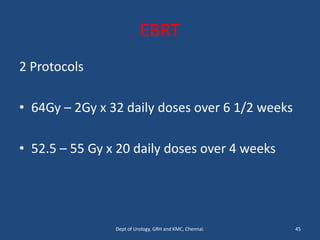
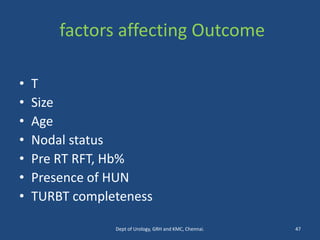
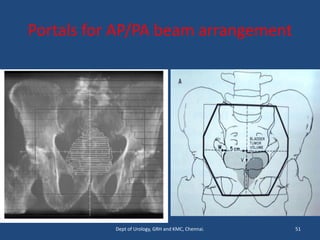
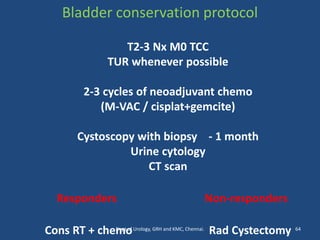
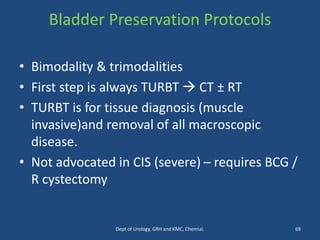
This document discusses the role of chemotherapy and radiotherapy in treating carcinoma of the bladder. It provides details on neoadjuvant chemotherapy, adjuvant chemotherapy, radical radiotherapy, and combined modality treatment for locally advanced disease. Neoadjuvant chemotherapy is found to improve survival outcomes compared to cystectomy alone by treating micrometastases. For metastatic bladder cancer, platinum-based regimens such as cisplatin and gemcitabine remain the standard first-line treatment. Radiotherapy can be used for organ-sparing treatment in select patients or as adjuvant therapy before or after surgery.