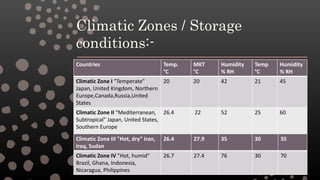
The document discusses drug stability and the factors that influence it. It defines stability as the extent to which a product retains its properties within specified limits throughout its shelf life. Drug stability depends on maintaining the physical, chemical, therapeutic and microbial properties of the dosage form over time. The purpose of stability studies is to determine shelf life and provide evidence of how quality varies with environmental factors like temperature and humidity. The types of stability that must be considered include chemical, physical, microbiological, therapeutic and toxicological stability. The document then discusses various types of degradation like physical, chemical and microbial and how they can be prevented. It also outlines ICH guidelines for stability testing.