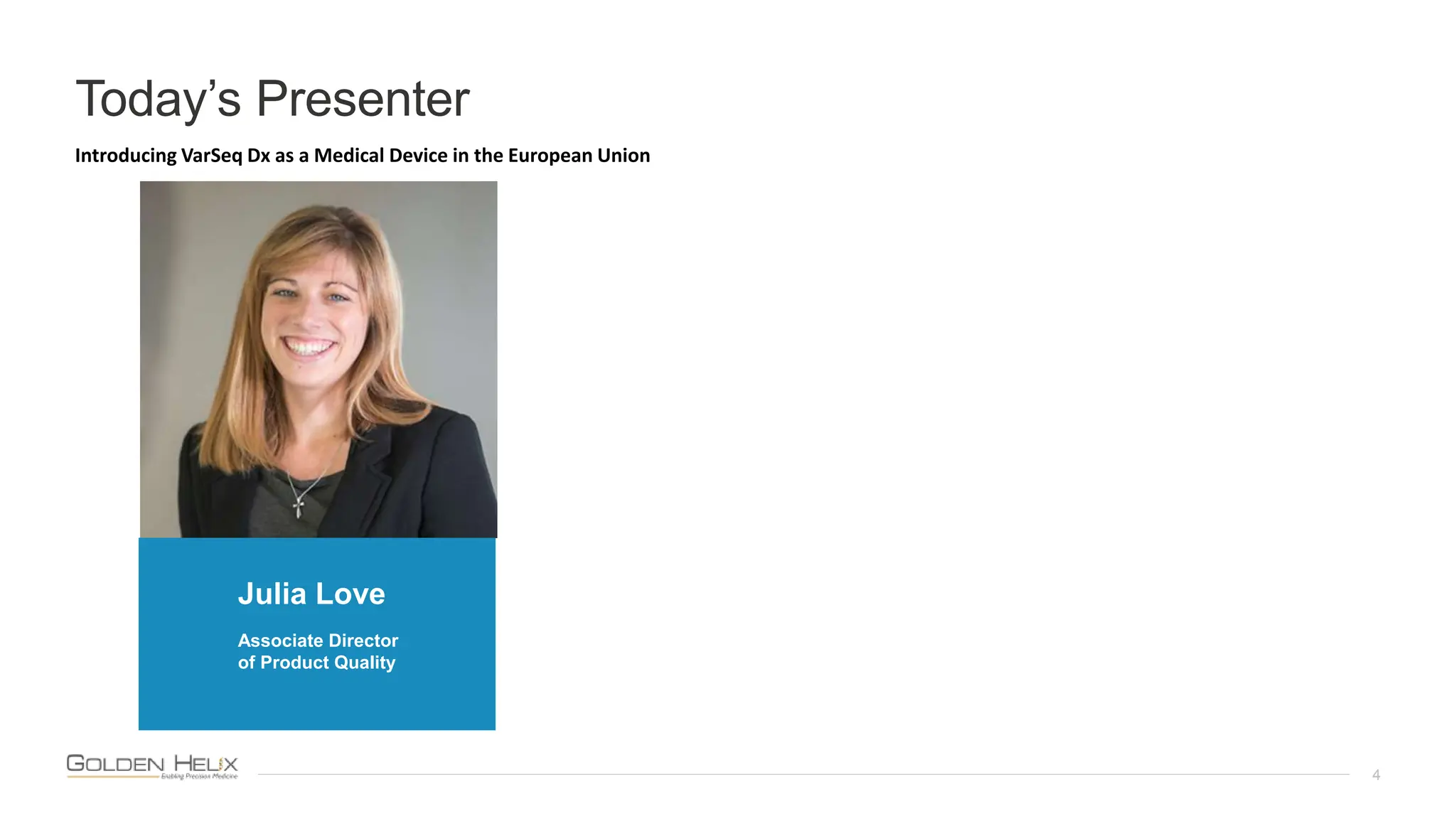
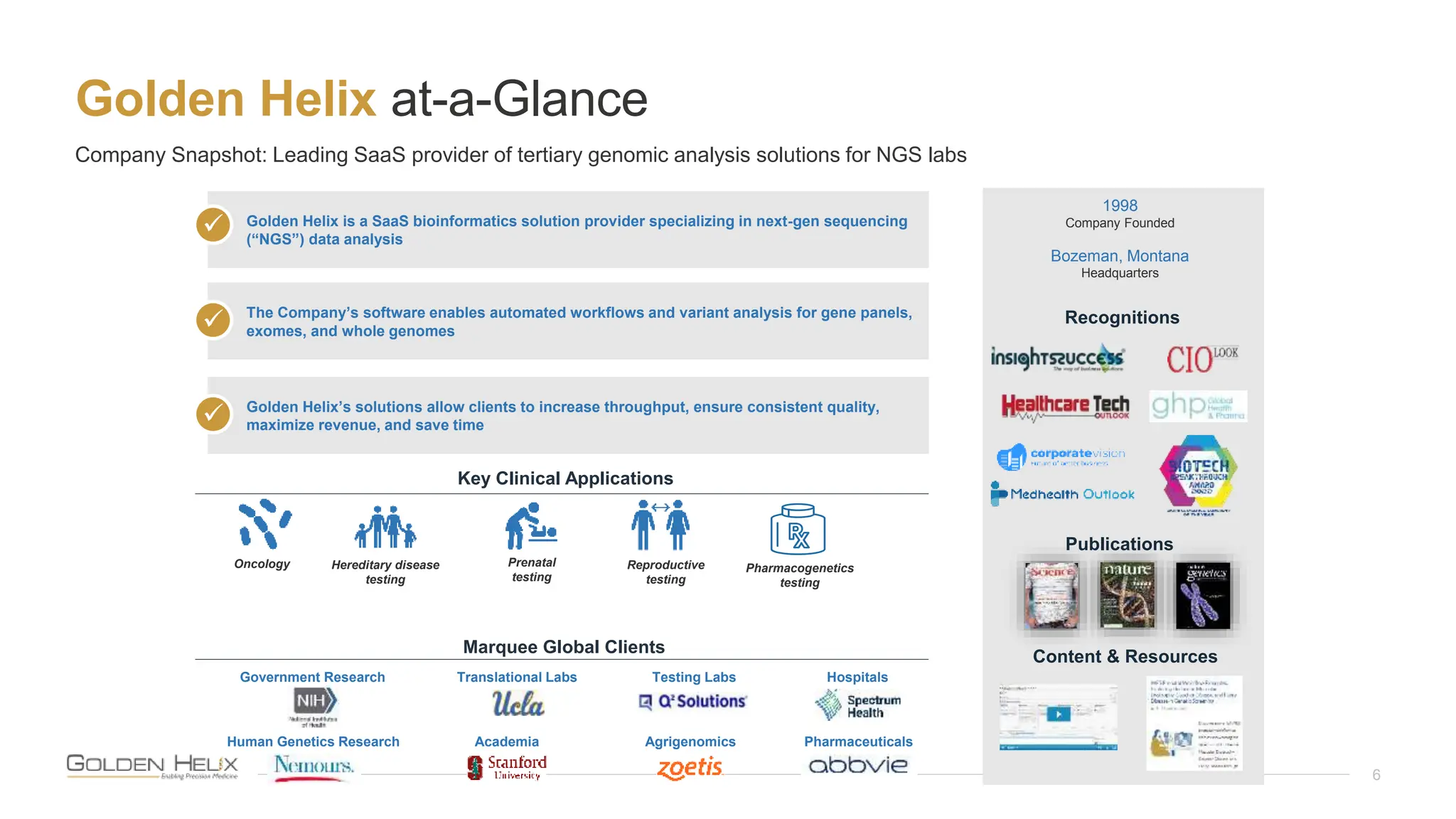
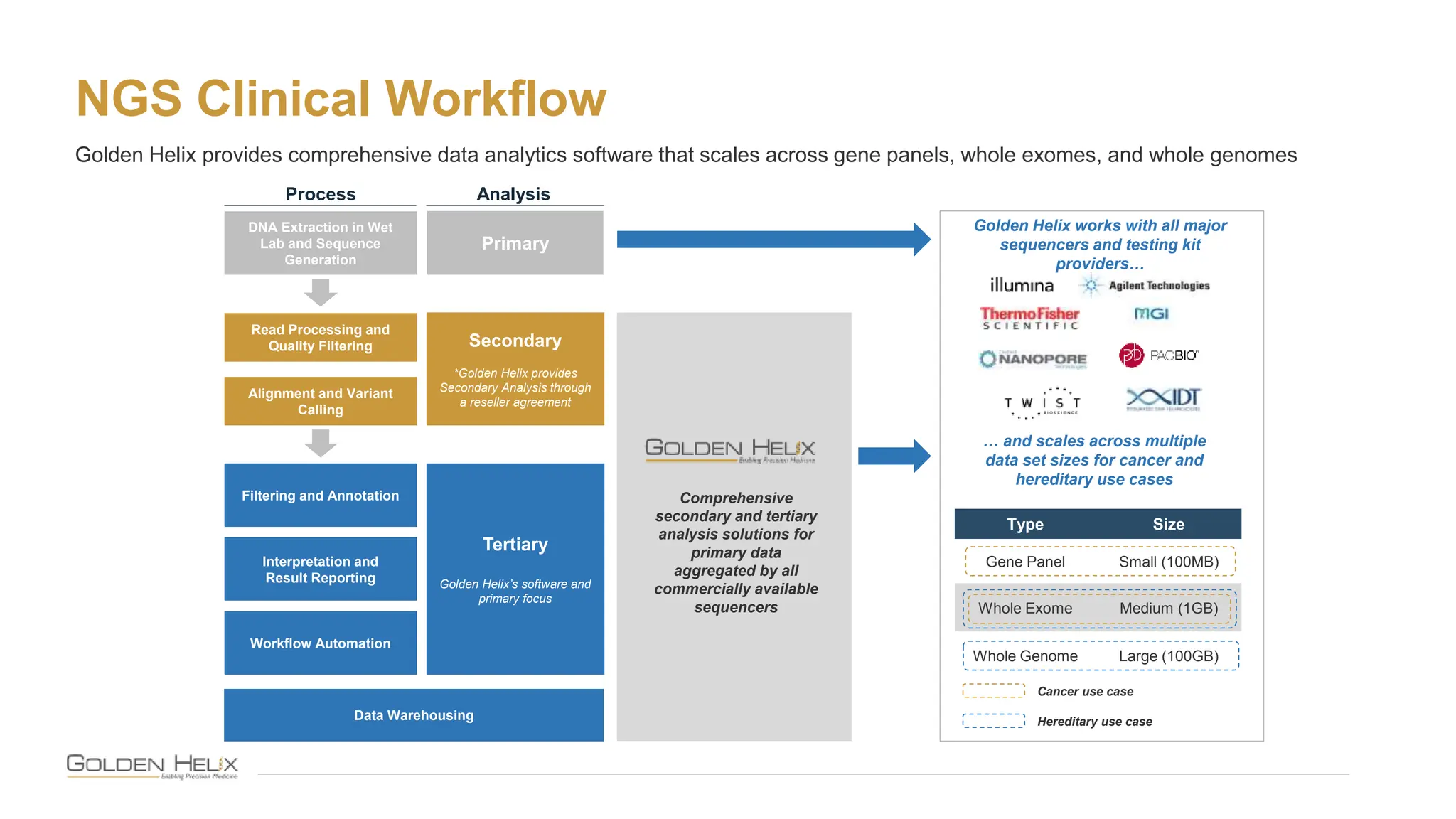
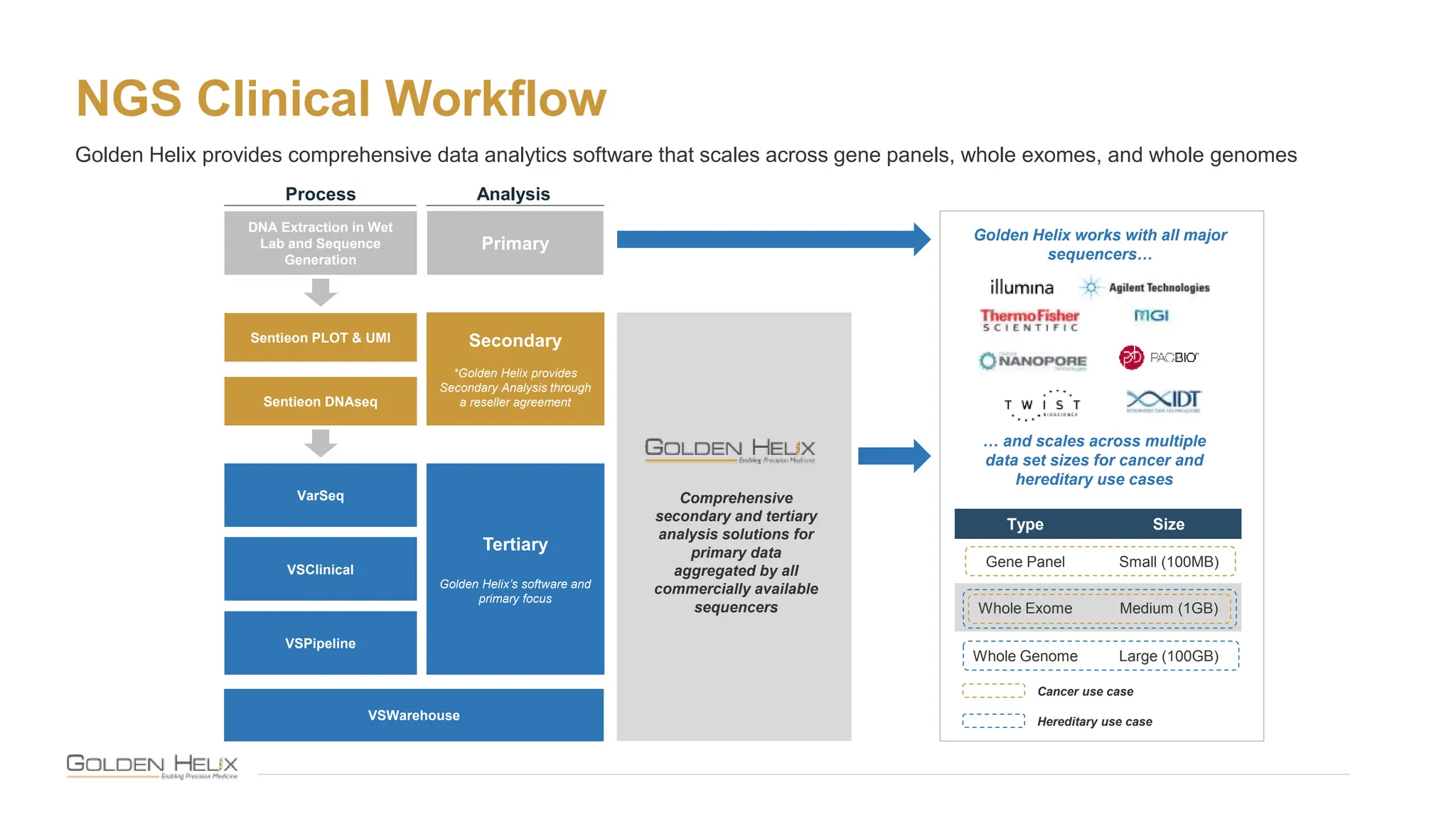
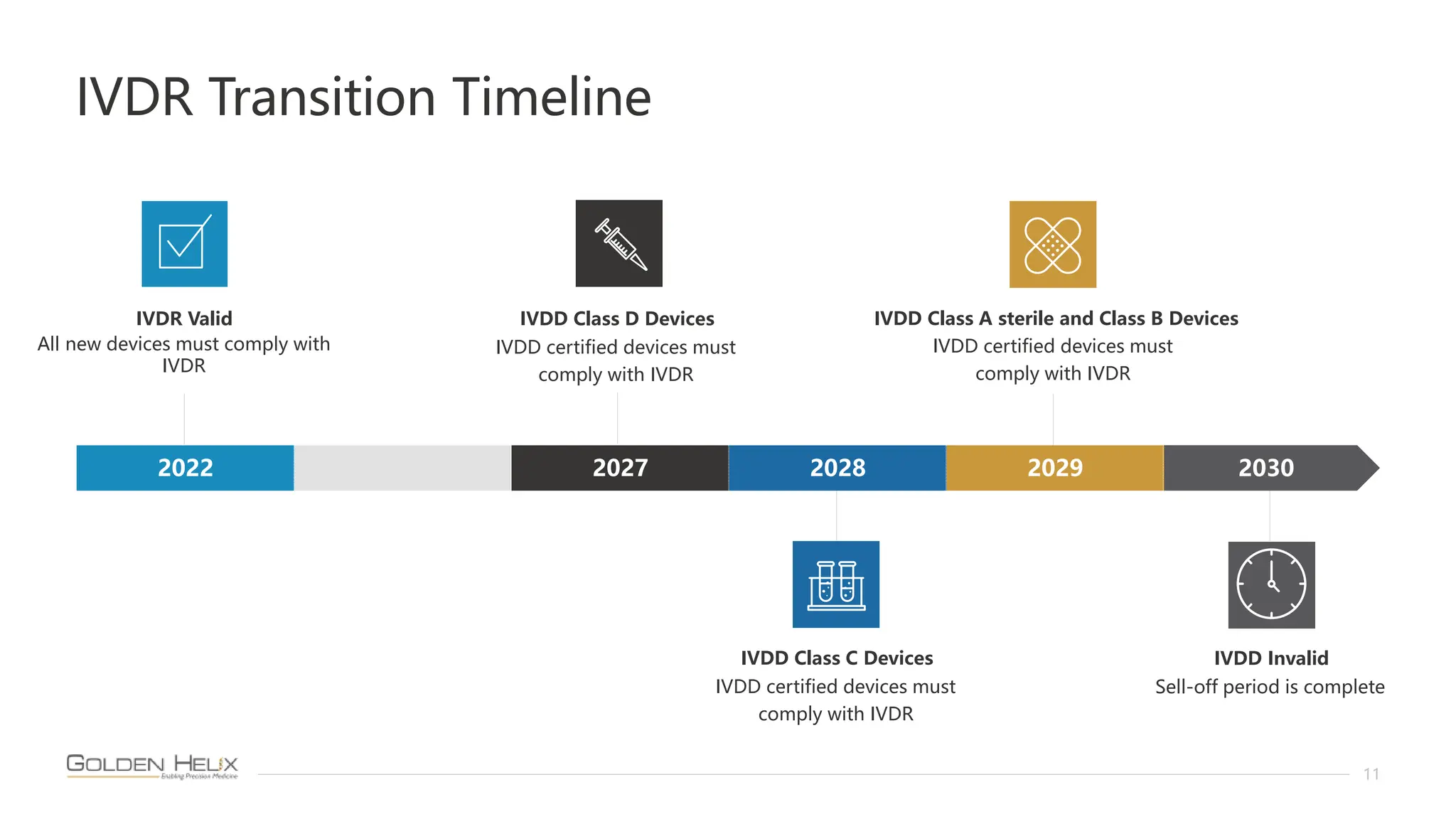
The document presents Golden Helix's introduction of VarSeq DX as a medical device in the EU, supported by NIH grants and meeting ISO 13485 and IVDR standards. This software aids in genomic data analysis for hospitals and labs, enhancing operational efficiency and compliance with medical device regulations. VarSeq DX mode, launched with version 2.6.1, requires verified installation and user certification for clinical use.