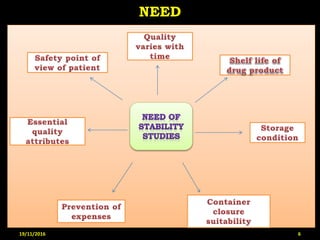
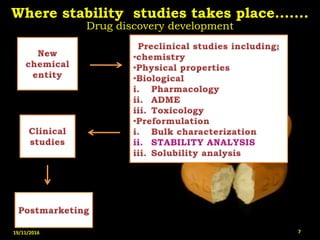
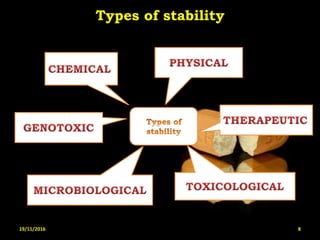
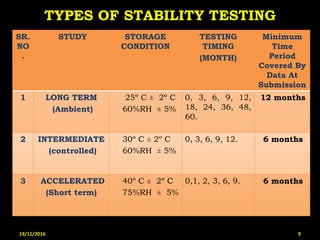
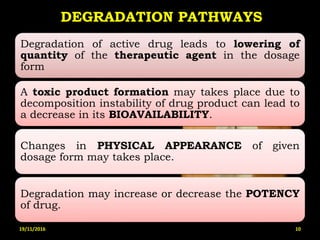
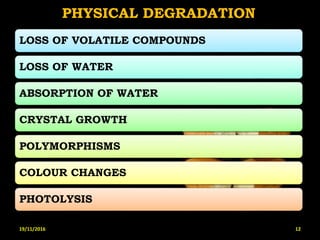
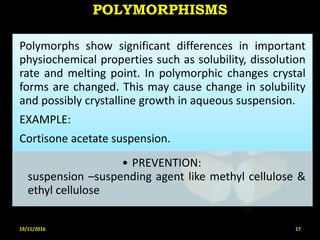
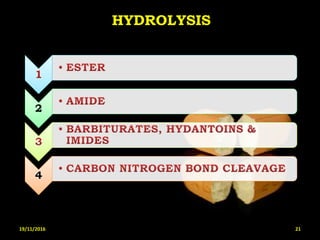
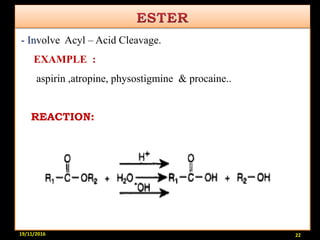
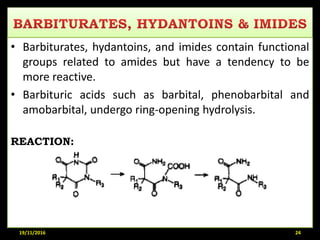
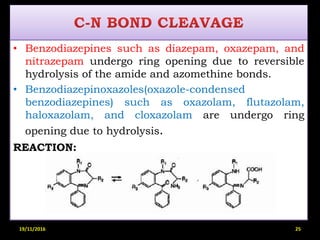
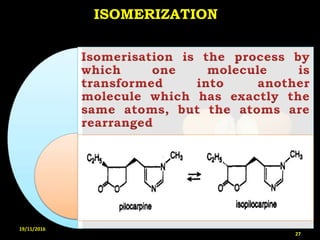
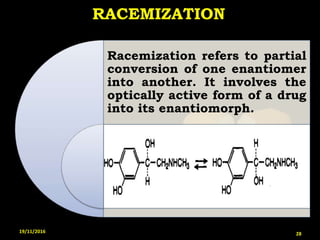
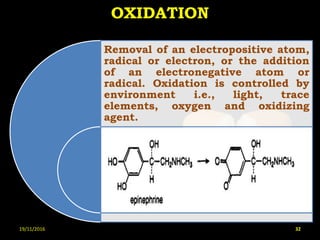
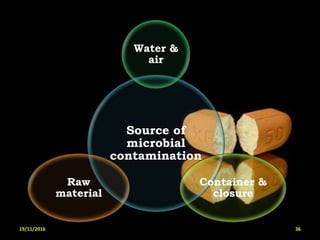
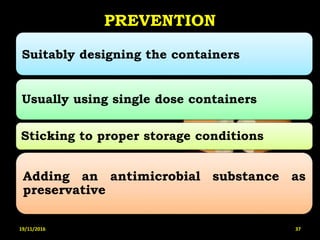
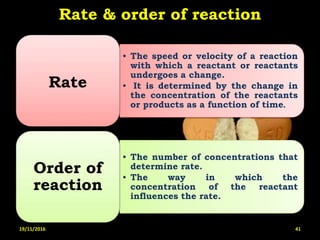
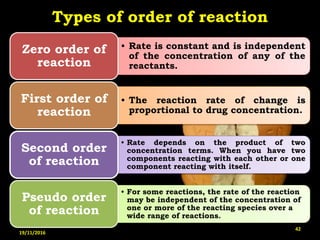
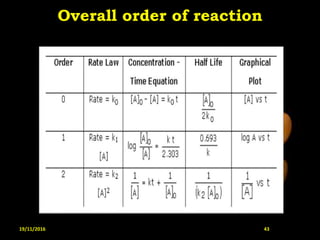
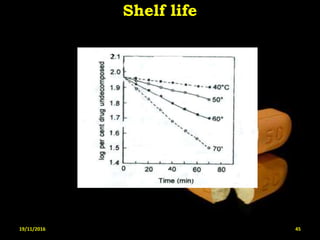
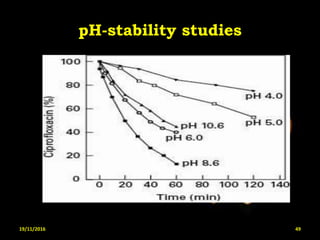
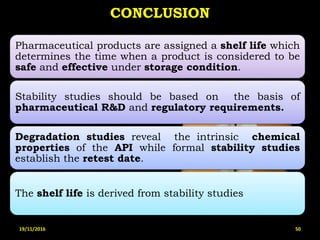
This document provides an overview of a seminar presentation on drug stability given by Ms. Swati S. Bharati to Mumbai University. The presentation covers topics such as the importance of stability testing, degradation pathways including physical, chemical and microbial degradation, kinetic stability, and solution and solid state stability. It defines stability and the purpose of stability studies. Examples are provided to illustrate different types of degradation pathways and how they can be prevented.