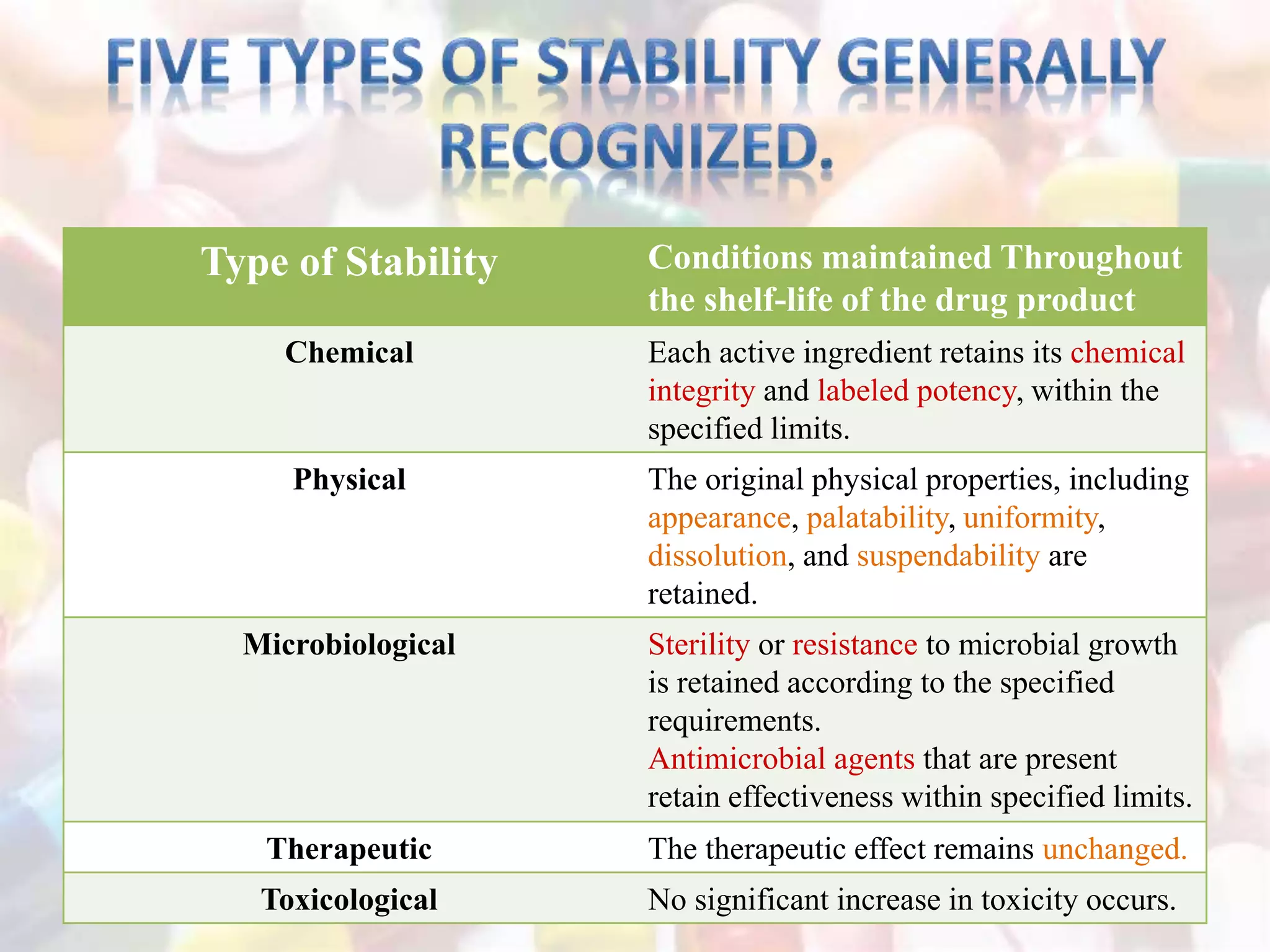
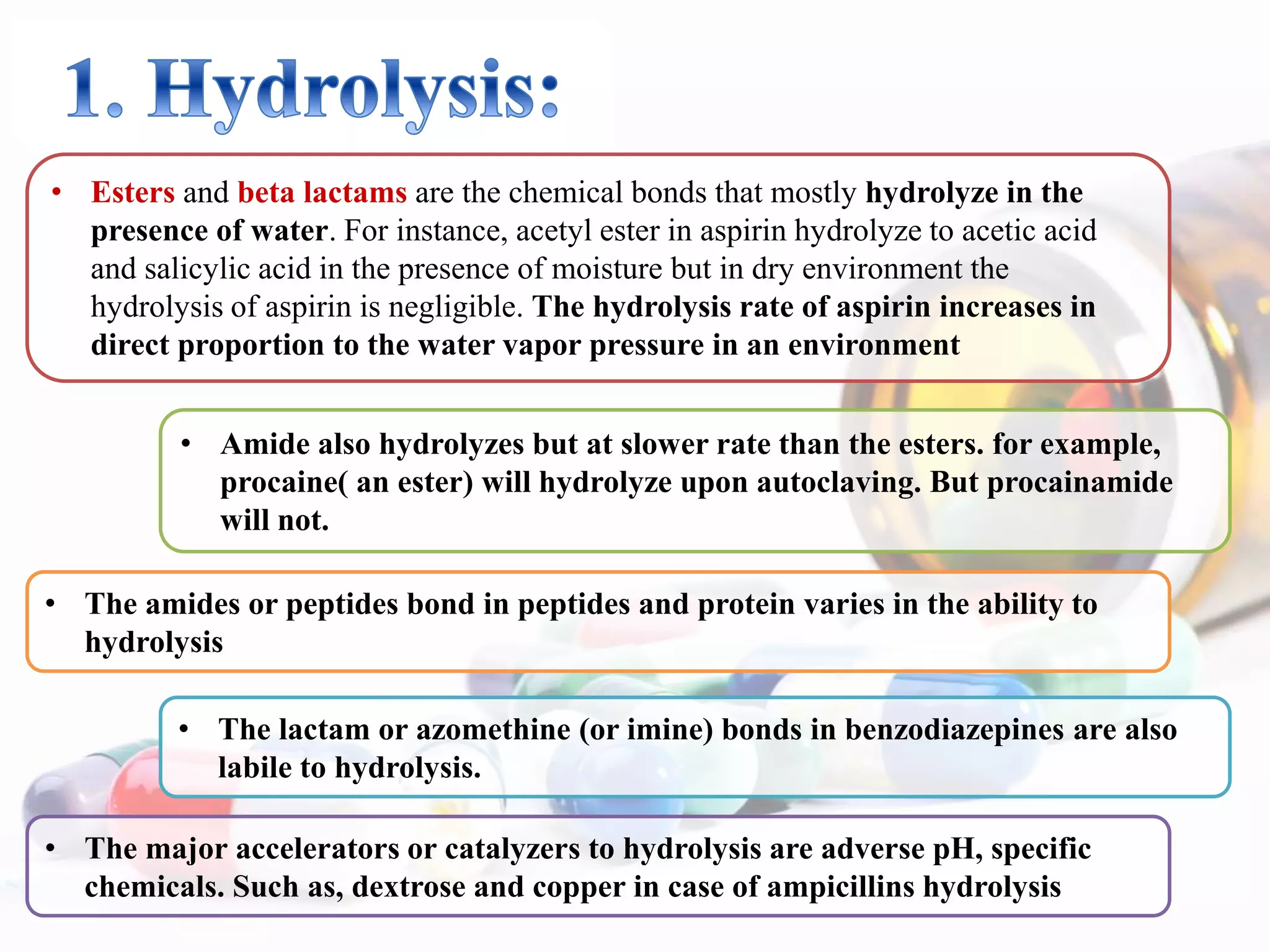
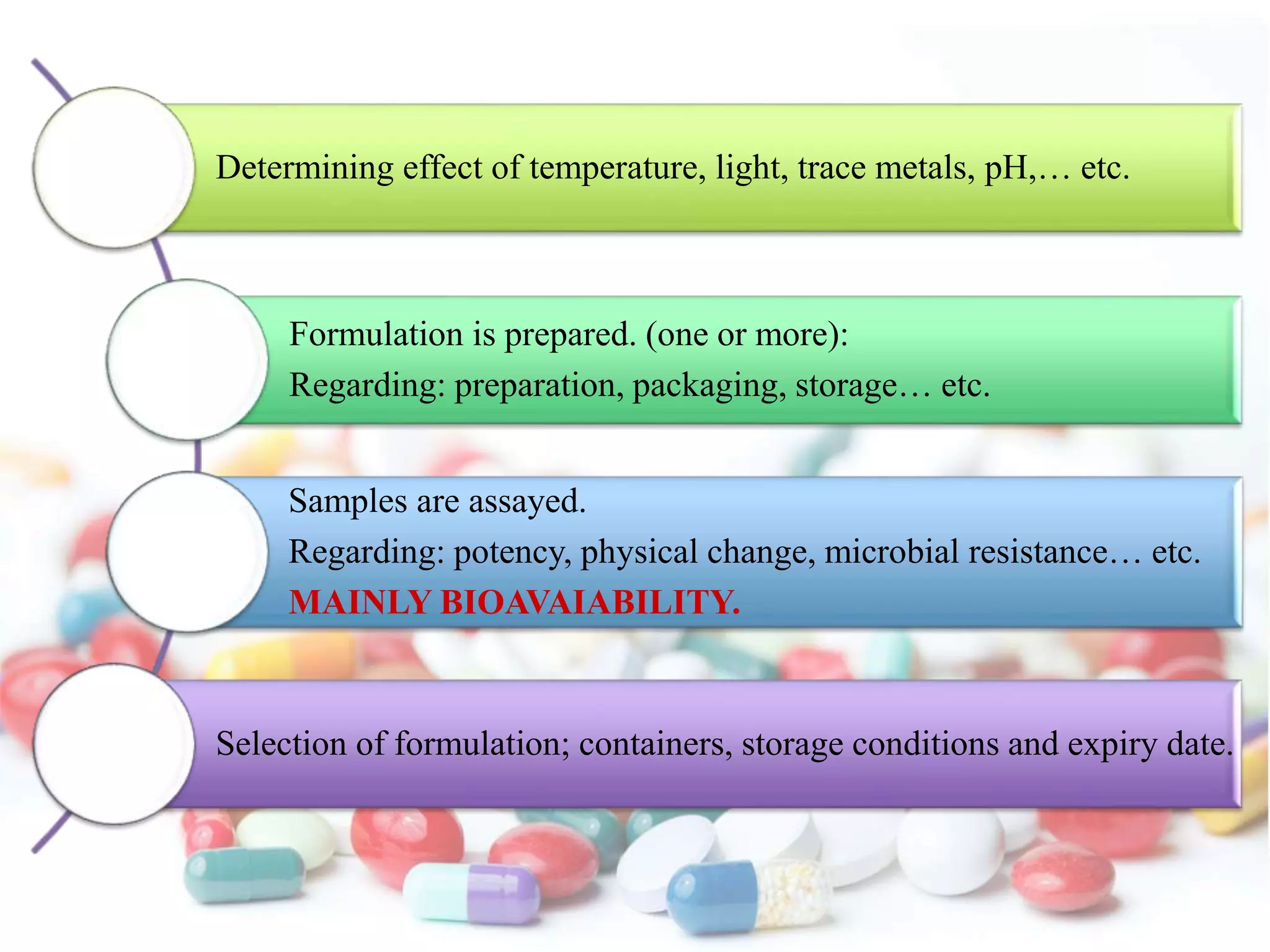
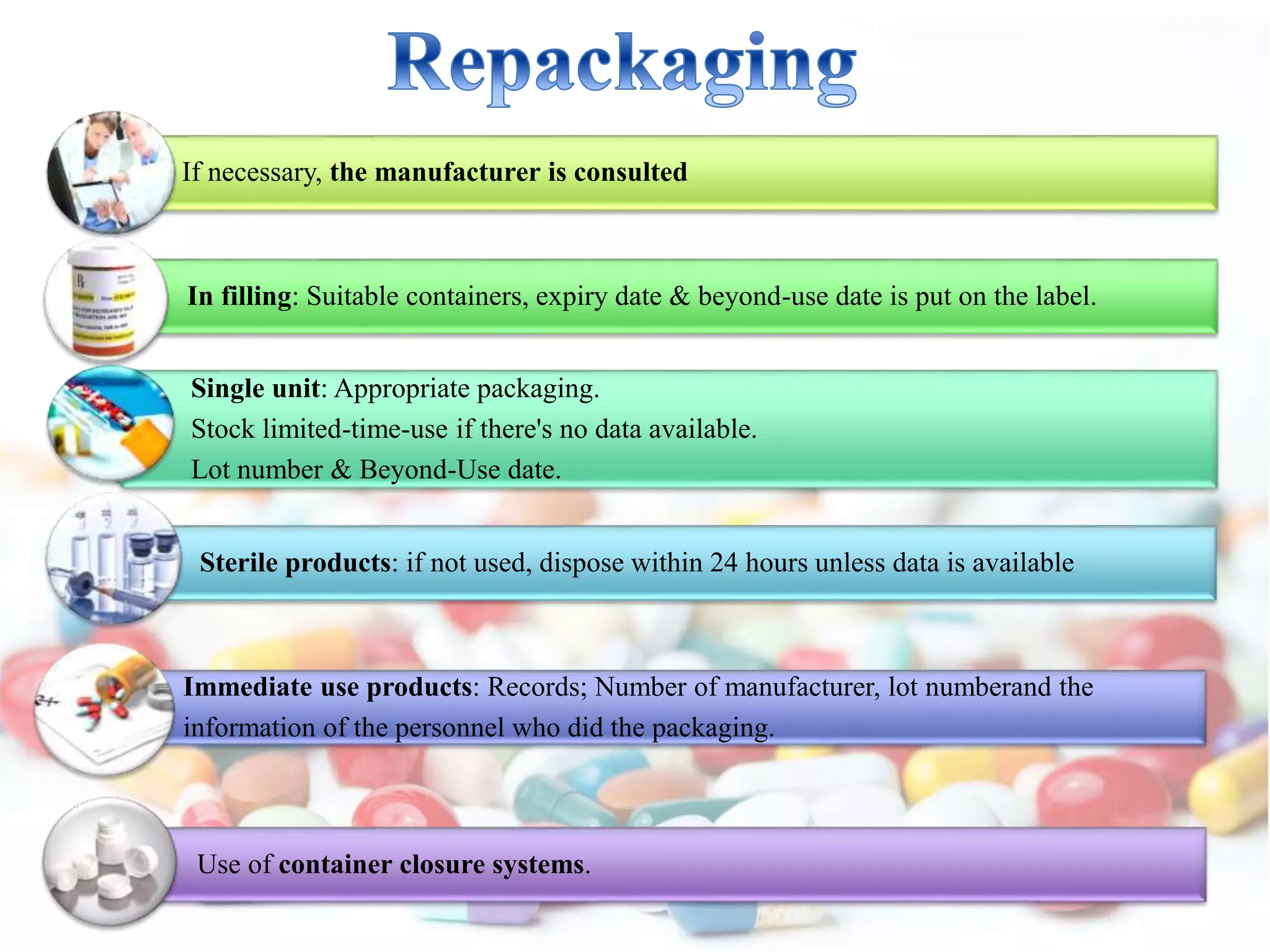
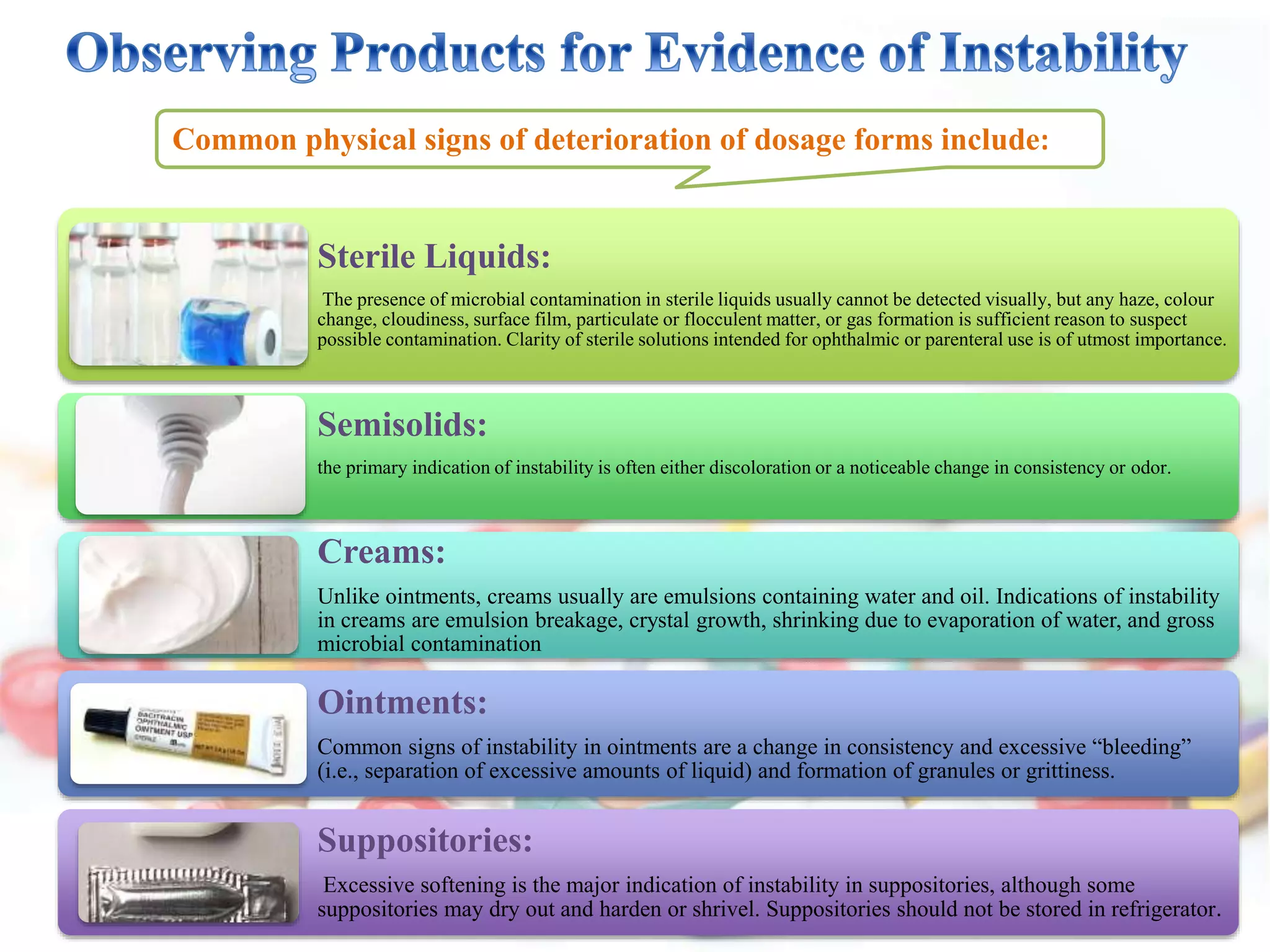
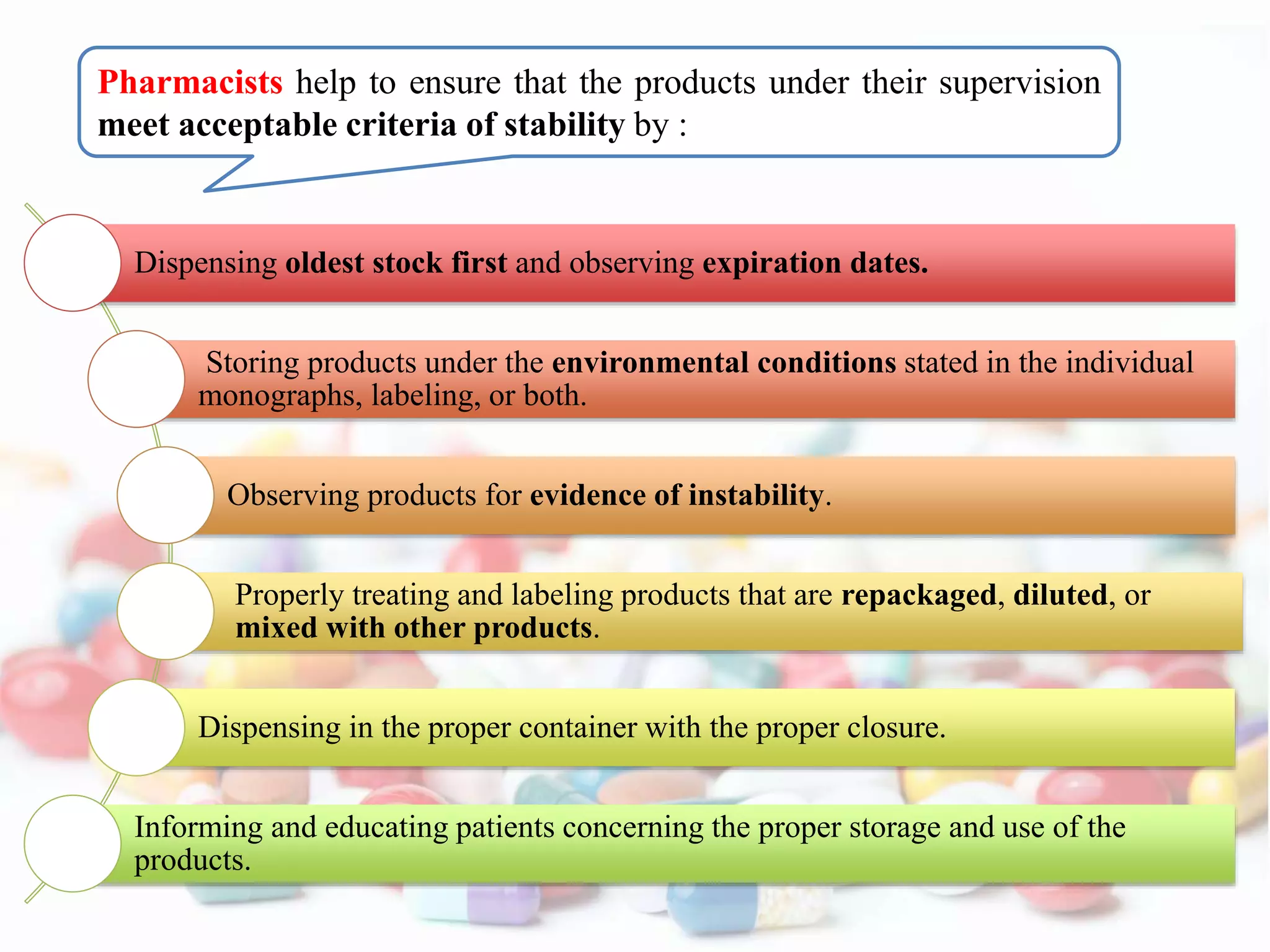
The document discusses the importance of stability in pharmaceutical compounding and outlines factors that can affect stability. It defines stability as a product retaining its properties and characteristics within specified limits throughout its shelf life. There are five main types of stability: chemical, physical, microbiological, therapeutic, and toxicological. Factors like temperature, light, humidity, ingredients, dosage form, pH, and solvent composition can influence stability. Pharmacists must store products under proper conditions and expiration dates to ensure stability and prevent issues.