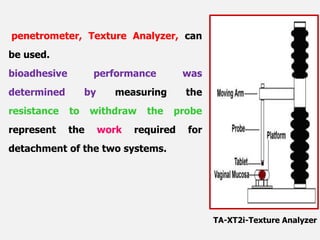
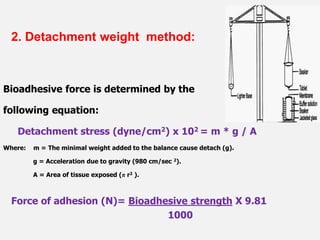
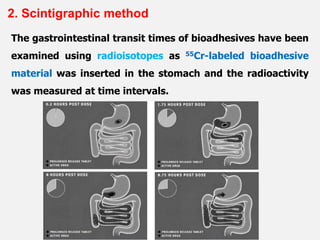
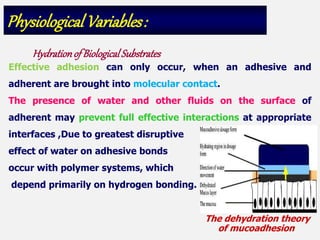
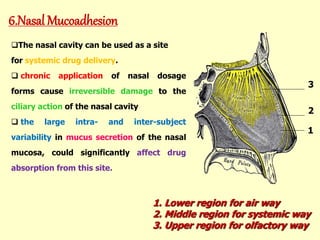
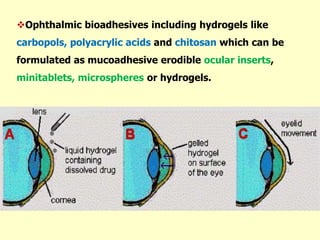
This document discusses the principles and mechanisms of bioadhesion and mucoadhesion for drug delivery systems, outlining how hydrophilic matrices like hydrogels can enhance drug contact time and localization at target sites. Various factors influencing bioadhesion, including polymer characteristics and the nature of biological substrates, are highlighted along with their implications for drug delivery via mucosal surfaces. The document also details methods for evaluating bioadhesive properties and applications in different delivery routes like buccal, gastric, and intestinal systems.