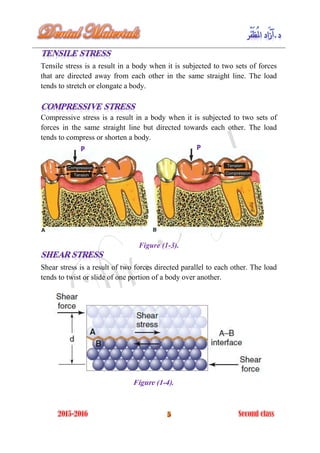
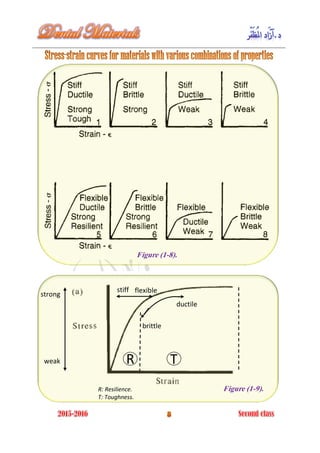
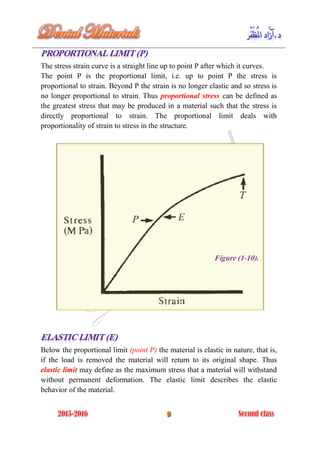
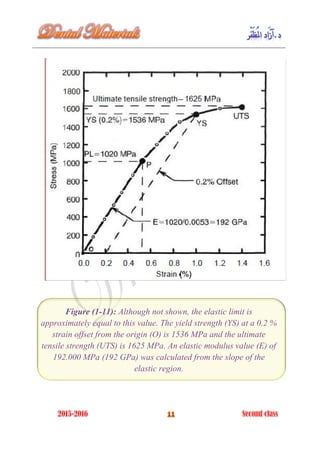
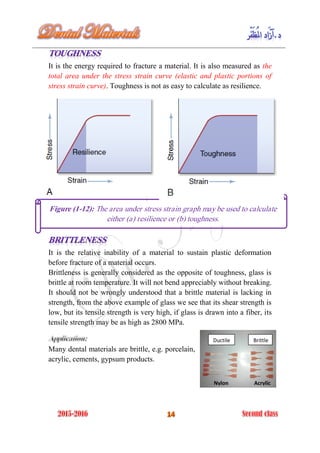
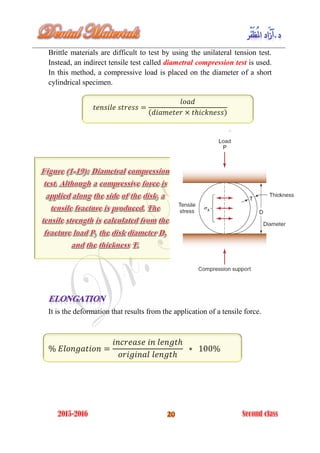
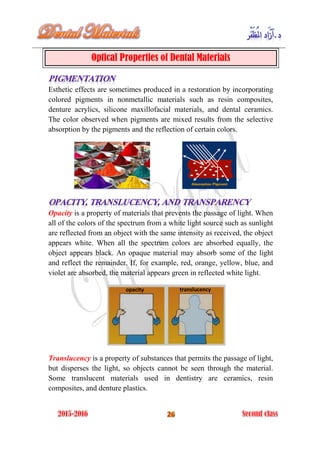
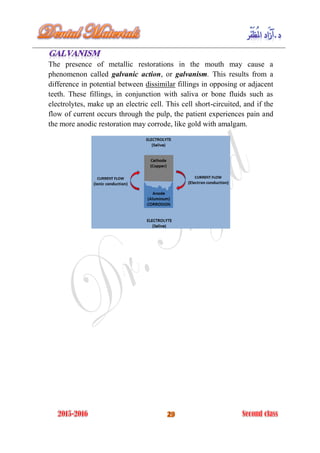
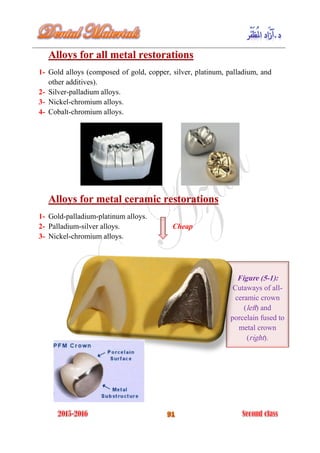
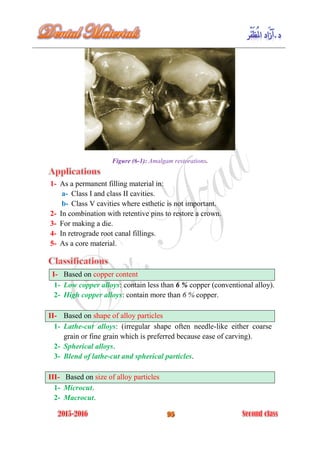
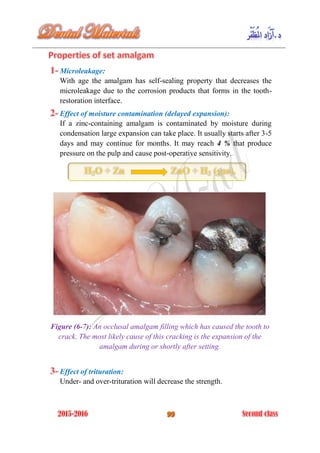
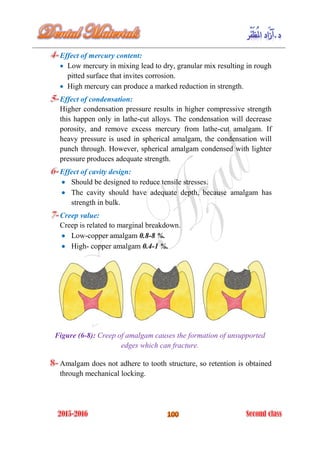
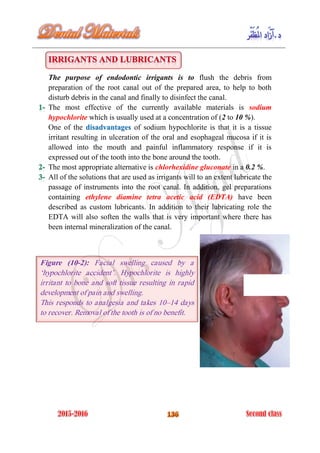
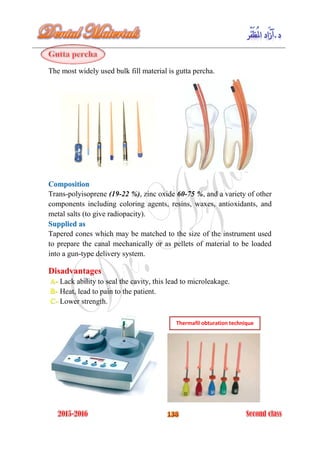
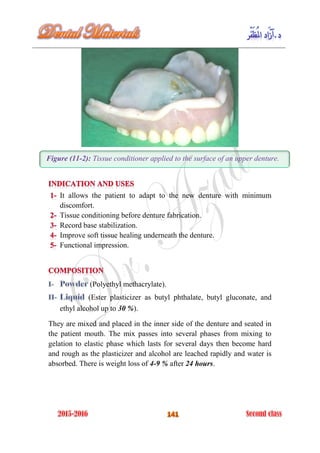
The document discusses the science of dental materials, covering their composition, properties, and interactions in the dental environment. It outlines various materials used for prevention, restoration, and rehabilitation in dentistry, as well as explains the physical and mechanical properties such as stress, strain, toughness, and hardness of these materials. Additionally, it details the classifications of atomic bonds and provides insights into the behavior of materials under different forces and conditions.