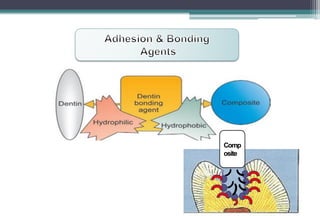
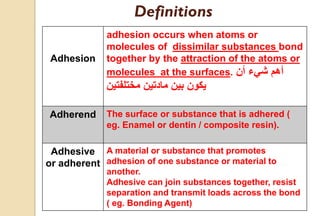
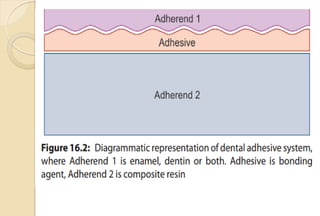
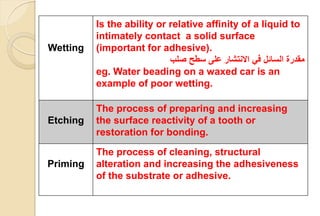
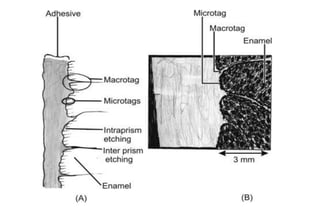
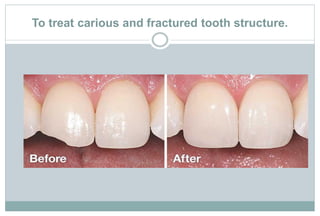
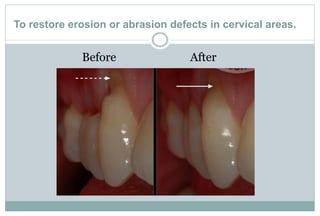
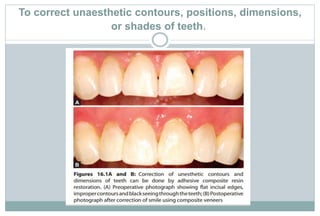
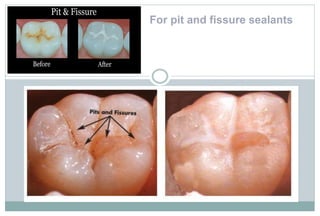
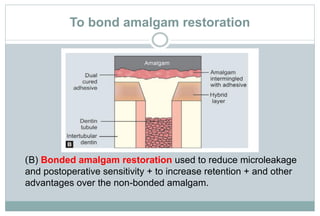
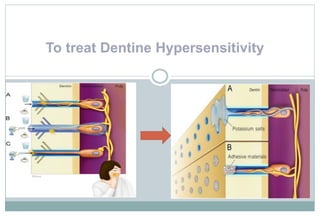
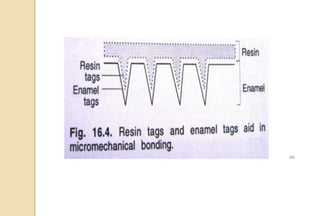
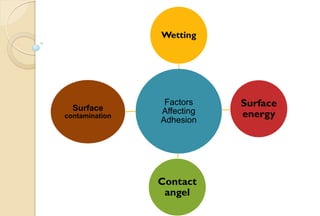
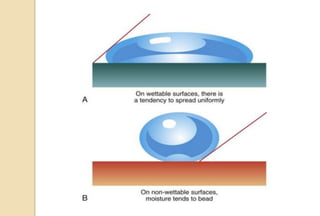
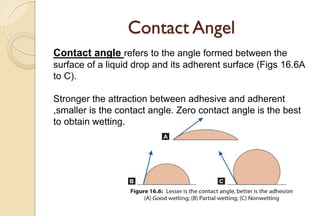
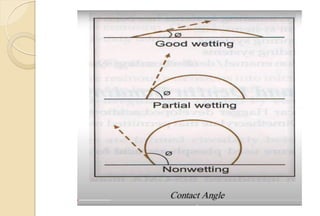
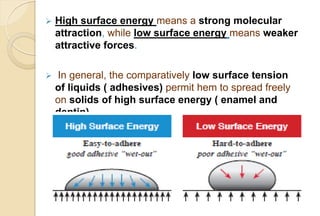
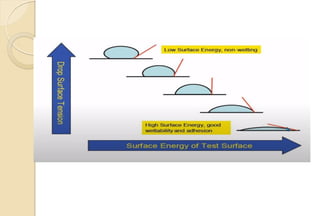
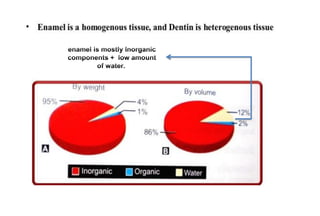
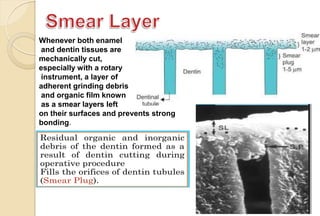
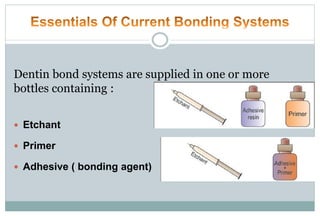
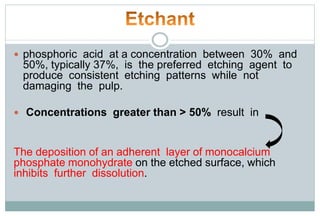
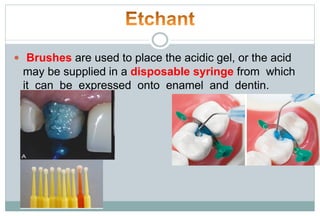
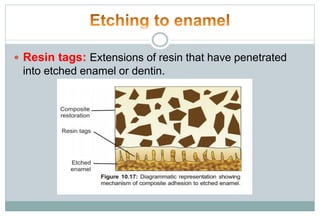
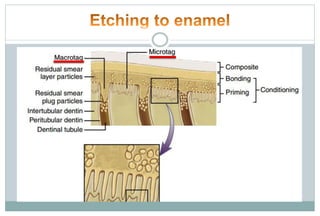
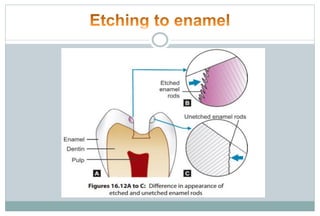
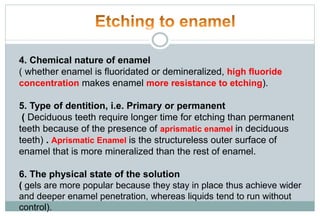
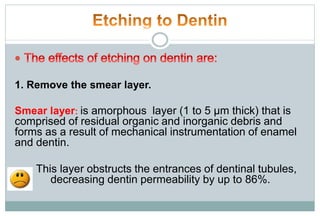
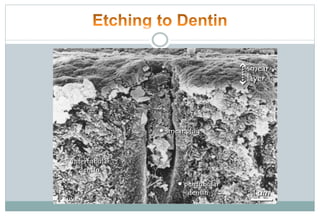
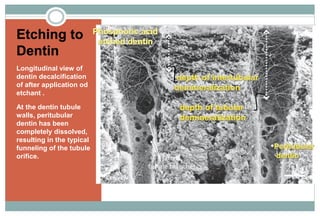
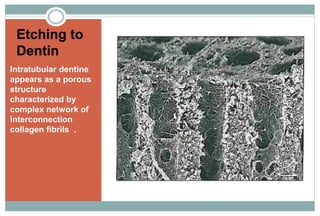
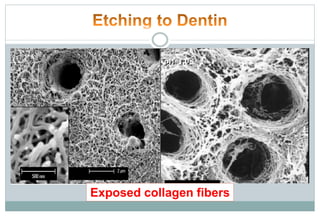
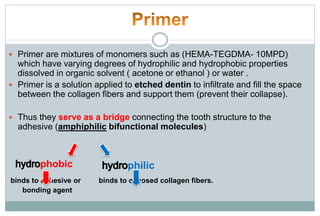
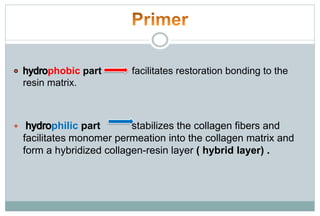
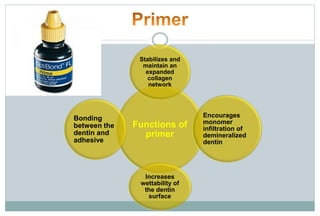
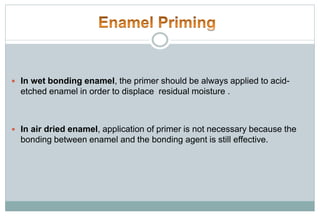
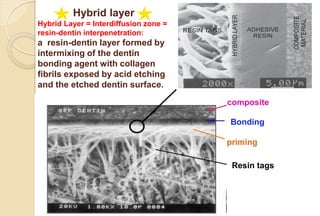
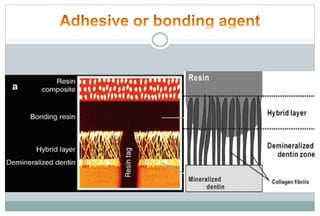
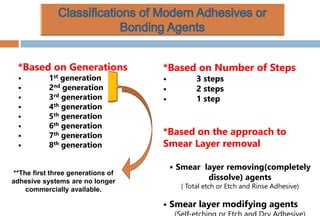
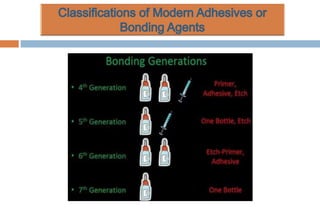
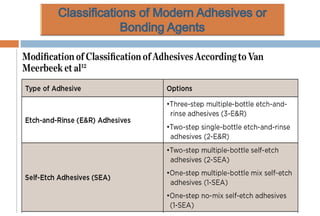
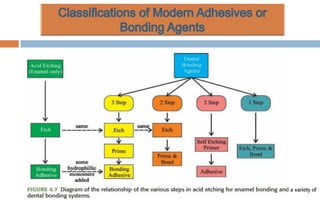
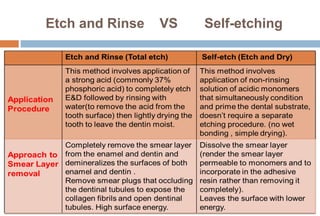
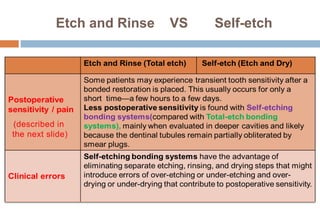
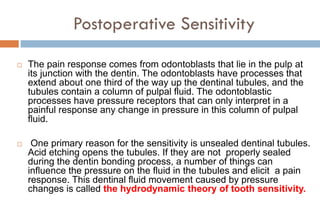
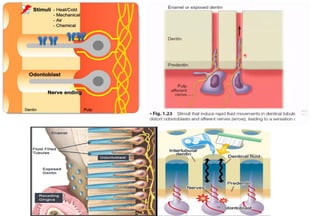
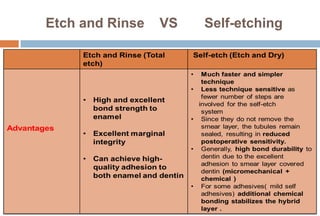
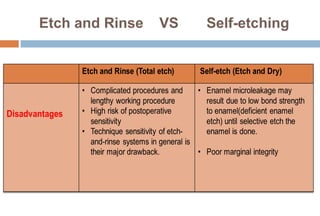
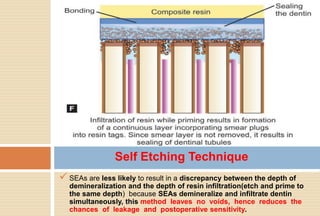
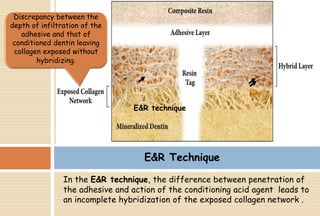
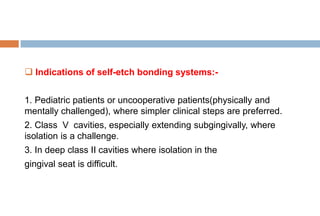
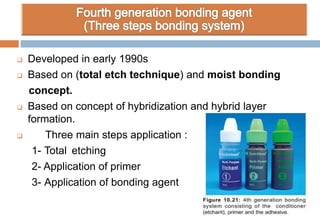
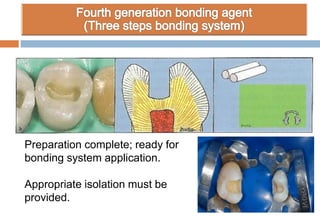
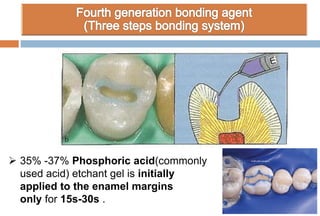
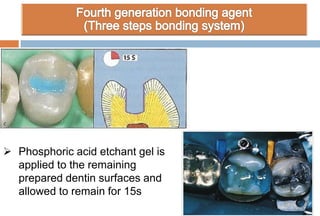
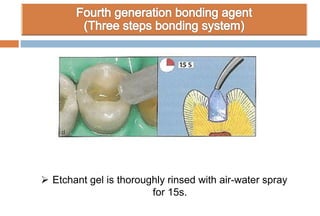
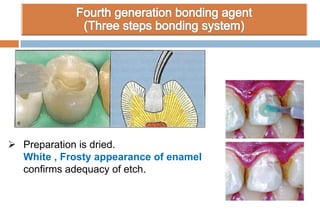
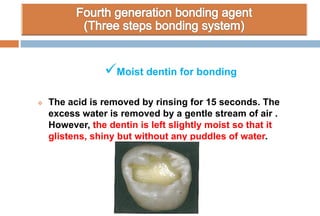
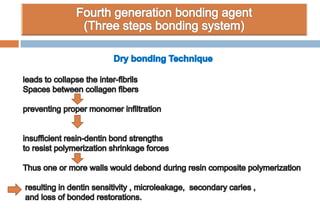
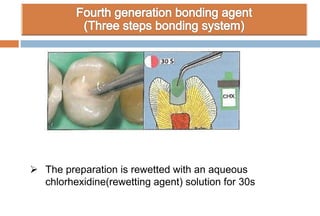
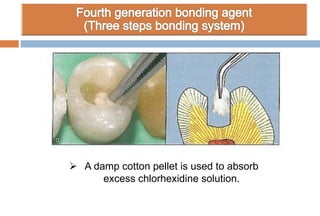
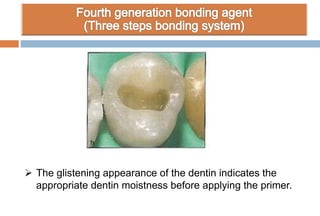
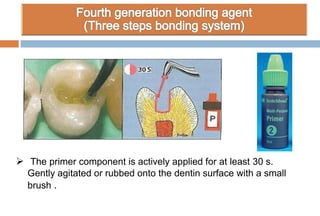
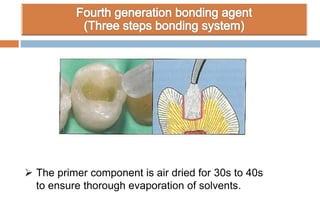
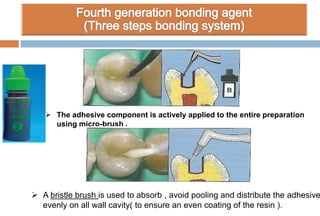
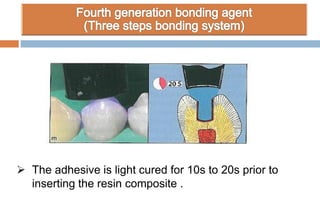
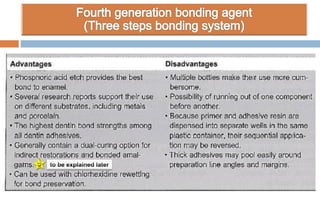
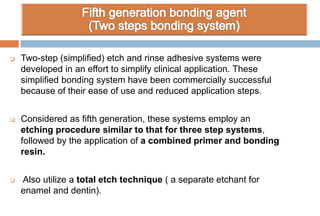
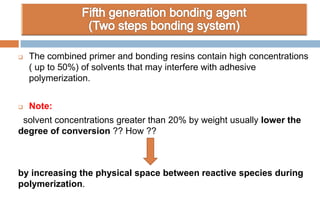
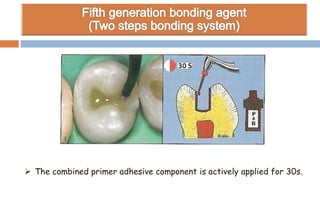
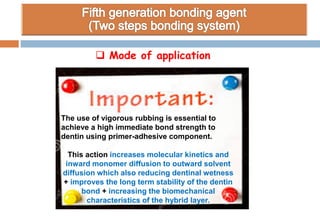
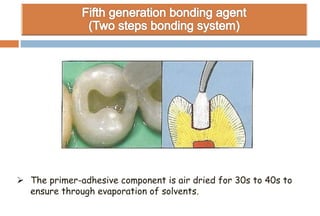
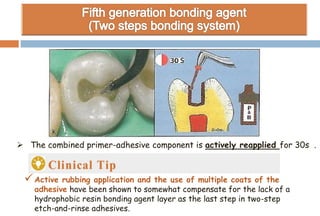
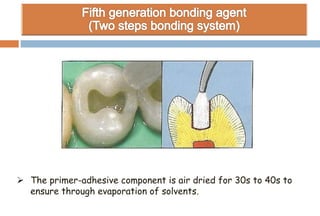
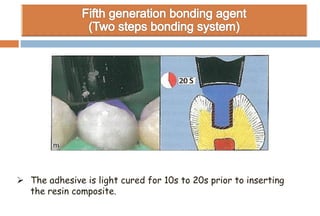
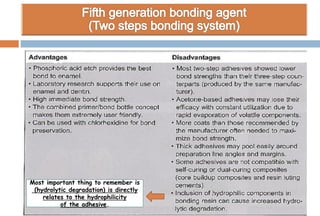
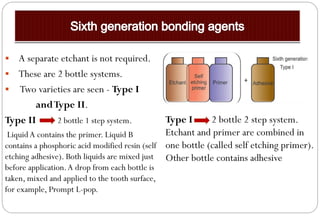
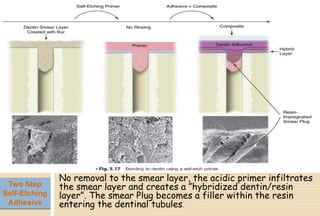
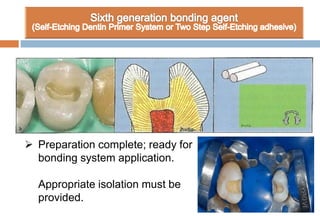
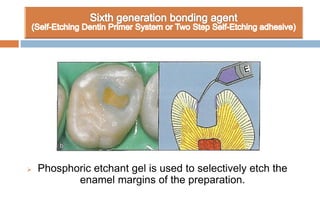
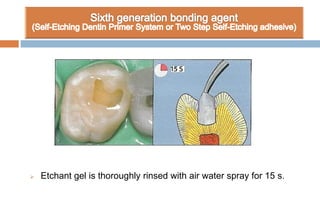
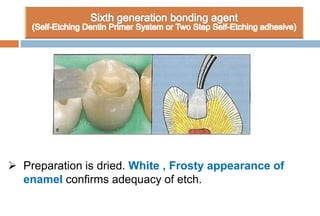
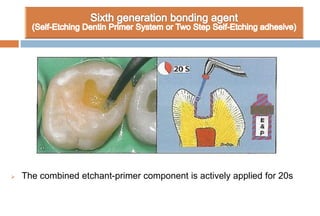
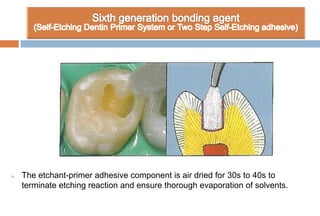
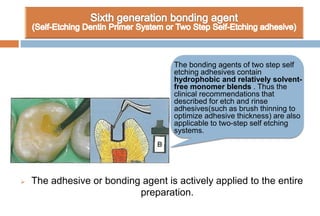
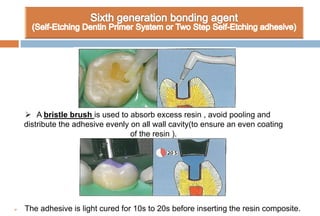
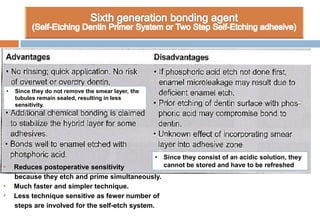
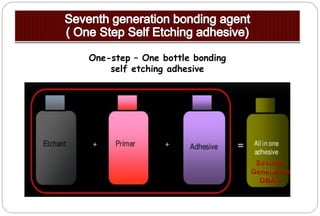
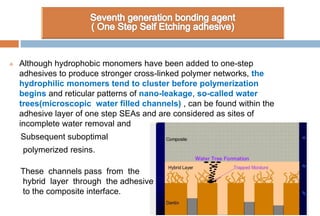
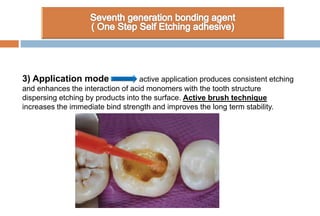
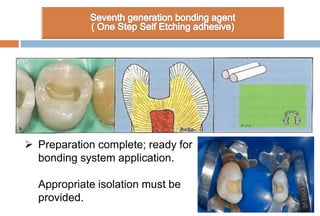
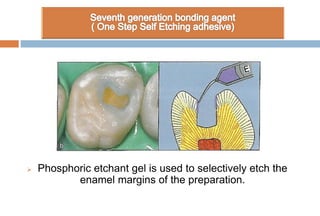
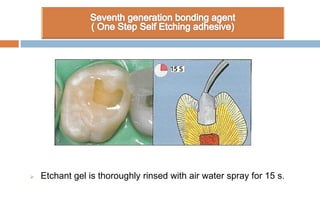
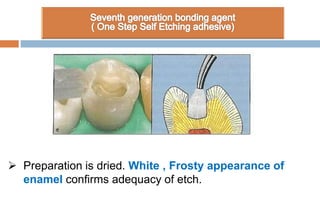
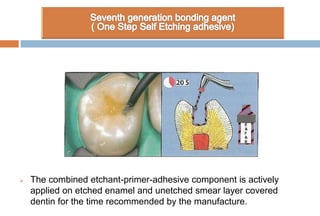
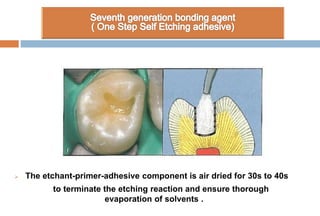
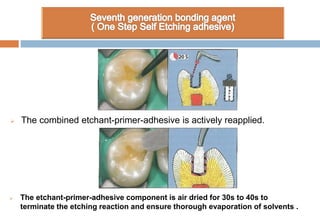
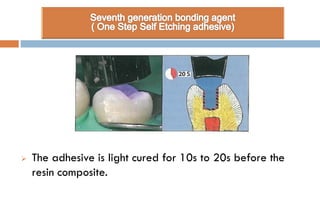
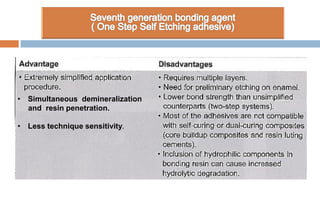
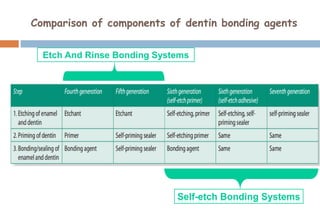
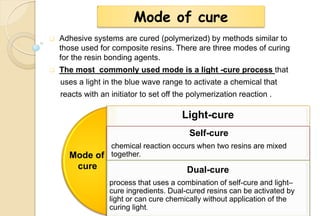
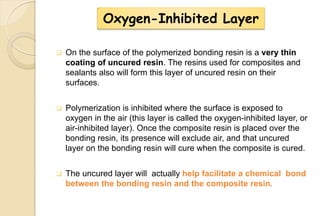
The document discusses the evolution and principles of adhesive dentistry, emphasizing the importance of adhesion for restorative materials to bond effectively to enamel and dentin. It outlines the mechanisms of adhesion, factors affecting it, and the components of dentin bonding systems, including etchants, primers, and bonding agents. The introduction of adhesive techniques has transformed restorative practices from traditional 'drill and fill' methods to more sophisticated approaches that require minimal tooth preparation.