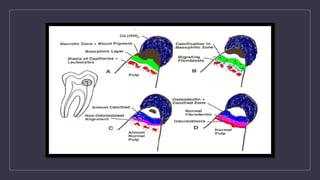
This document discusses calcium hydroxide, including its classification, properties, mechanisms of action, and applications. Calcium hydroxide can be classified based on setting time or mechanism of setting. It has high pH and alkalinity which provides its therapeutic effects such as antibacterial action and stimulation of hard tissue formation. Its main applications include pulp capping, pulpotomy, apexification, and as an intracanal medicament. It promotes healing through formation of a zone of coagulation necrosis followed by a dentinal bridge. Overall, calcium hydroxide is a widely used material in endodontics due to its biocompatibility and ability to stimulate hard tissue repair.